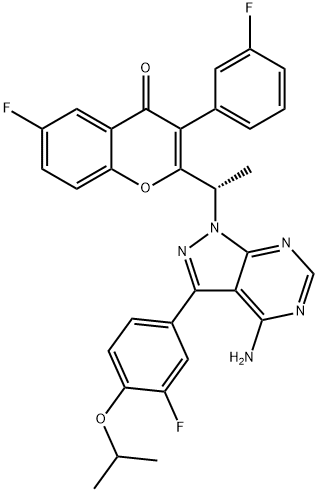
Umbralisib synthesis
- Product Name:Umbralisib
- CAS Number:1532533-67-7
- Molecular formula:C31H24F3N5O3
- Molecular Weight:571.55
To a solution of intermediate 13 (0.134 g, 0.494 mmol) in THF (2.0 ml), intermediate 5 (0.150 g, 0.494 mmol) and triphenylphosphine (0.194 g, 0.741 mml) were added and stirred at RT for 5 min. Diisopropylazodicarboxylate ( 0.15 ml, 0.749 mmol) was added heated to 45°C. After 2h, the reaction mixture was quenched with with water and extracted with ethyl acetate. The organic layer was dried over sodium sulphate and concentrated under reduced pressure. The crude product was purified by column chromatography with ethyl acetate : petroleum ether to afford Umbralisib as an off-white solid (0.049 g, 20 %).
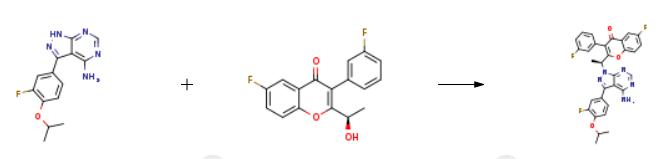
2105-94-4
346 suppliers
$6.00/5g

1532533-67-7
76 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 4 steps
1.1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 1 h / 45 °C / Reflux
2.1: potassium acetate / 1,4-dioxane / 0.5 h
2.2: 12 h / 80 °C / Inert atmosphere
3.1: sodium carbonate / water; N,N-dimethyl-formamide; ethanol / 0.5 h
3.2: 12 h / 80 °C / Inert atmosphere
4.1: triphenylphosphine / tetrahydrofuran / 0.08 h / 25 - 28 °C / Reflux
4.2: 2 h / 45 °C
References:
RHIZEN PHARMACEUTICALS SA;VAKKALANKA, Swaroop Kumar Venkata Satya;MUTHUPPALANIAPPAN, Meyyappan;NAGARATHNAM, Dhanapalan WO2014/6572, 2014, A1
202865-80-3
69 suppliers
$32.00/1g

1532533-67-7
76 suppliers
inquiry
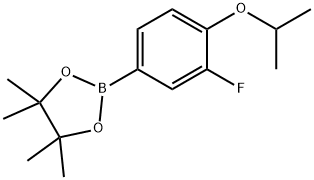
1350426-06-0
30 suppliers
inquiry

1532533-67-7
76 suppliers
inquiry
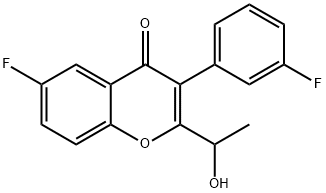
1532533-31-5
3 suppliers
inquiry

1532533-67-7
76 suppliers
inquiry

1300582-10-8
4 suppliers
inquiry

1532533-67-7
76 suppliers
inquiry