Toltrazuril synthesis
- Product Name:Toltrazuril
- CAS Number:69004-03-1
- Molecular formula:C18H14F3N3O4S
- Molecular Weight:425.38
![Imidodicarbonic diamide, 2-methyl-N-[3-methyl-4-[4-[(trifluoromethyl)thio]phenoxy]phenyl]-](/CAS/20210111/GIF/106310-18-3.gif)
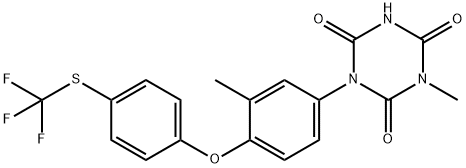
106310-18-3
0 suppliers
inquiry
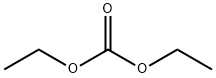
105-58-8
447 suppliers
$15.00/25g

69004-03-1
374 suppliers
$5.00/250mg
Yield:69004-03-1 81%
Reaction Conditions:
Stage #1: 3-methyl-1-[3-methyl-4-(4-(trifluoromethylsulfanyl)phenoxy)phenyl]biuret;Diethyl carbonatewith sodium methylate in toluene at 50; for 4 h;
Stage #2: with acetic acid in water;toluene at 70; pH=7;Reagent/catalyst;Solvent;Temperature;
Steps:
10; 11; 12 Preparation of Toltrazuril
In a 500mL round bottom flask, add 60g of diethyl carbonate (5eq), 12.0g of sodium hydride (3eq, sodium hydride stored in paraffin, content about 60%), stir and mix well, and The temperature was raised to 60°C, and 34.8 g of 3-methyl-1-[3-methyl-4-(4-trifluoromethylthiophenoxy)phenyl]-biuret prepared in Example 8 was added dropwise , Dissolve in 75mL of toluene, dropwise addition time is 90min, drop to completion, keep warm for 3h, cool to room temperature, slowly add acetic acid (30mL), adjust system pH=7, then slowly add water (90mL), the system is light yellow and turbid , Raise the temperature to 70 , dissolve and clarify the system, stand still and separate the layers, separate the liquid, take the organic layer, then extract the aqueous layer with toluene (50mL×2), and take the organic layer, combine the organic layers, and evaporate the organic solvent under reduced pressure To obtain a pale yellow totrastuzil crude solid, and recrystallized with methanol to obtain 35.1g of pure product, the molar yield was 95%, the content measured by HPLC was 99.6%, and the total yield was 81%.
References:
CN111153863,2020,A Location in patent:Paragraph 0047-0053
![N-methyl-N-[3-methyl-4-[4-[(trifluoromethyl)thio]phenoxy]phenyl]-Imidodicarbonic diamide](/CAS/20180703/GIF/106310-17-2.gif)
106310-17-2
5 suppliers
inquiry

105-58-8
447 suppliers
$15.00/25g

69004-03-1
374 suppliers
$5.00/250mg
![3-methyl-4-[4-[(trifluoromethyl)thio]phenoxy]benzenamine](/CAS/20180702/GIF/94155-78-9.gif)
94155-78-9
8 suppliers
inquiry

69004-03-1
374 suppliers
$5.00/250mg