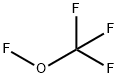
TRIFLUOROMETHYLHYPOFLUORITE synthesis
- Product Name:TRIFLUOROMETHYLHYPOFLUORITE
- CAS Number:373-91-1
- Molecular formula:CF4O
- Molecular Weight:104
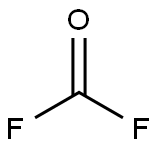
353-50-4
1 suppliers
inquiry

373-91-1
11 suppliers
inquiry
Yield: 98%
Reaction Conditions:
copper;cesium fluoride at 100;
Steps:
A
EXAMPLES; Example A; Synthesis of CF3OF; 10 l/h of gaseous fluorine, 5 l/h of CO and 10 l/h of nitrogen are contemeporaneously allowed to flow in an AISI 316 steel pipe (inner diameter 2.17 mm and length 100 mm). The reaction is triggered by heating the gas mixing zone at 100° C. for some minutes. During the whole time the reactor is cooled by air circulation so that the temperature is lower than 300° C.; at the reactor outlet the temperature is 250° C. Under these conditions CO and F2 are converted into COF2 with a yield higher than 95% (determined by IR analysis of the outflowing gaseous mixture).The gaseous mixture, after cooling at 100° C., is allowed to flow through a catalytic bed formed of 300 g of fine milled anhydrous CsF having particle size lower than or equal to 0.1 mm, mixed with 300 g of needle-shaped copper having diameter of 0.2 mm and length 6-7 mm. The catalyst is placed in a tubular reactor (inner diameter 55 mm, length 250 mm). The reaction temperature among gases is maintained at 100° C. Under these conditions the COF2 is converted into CF3OF with yield higher than 98%, determined by IR analysis of the outflowing mixture.
References:
SOLVAY SOLEXIS S.p.A. US2007/149827, 2007, A1 Location in patent:Page/Page column 4

124-38-9
122 suppliers
$175.00/23402

373-91-1
11 suppliers
inquiry
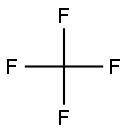
75-73-0
81 suppliers
$145.00/100 g

353-50-4
1 suppliers
inquiry

353-50-4
1 suppliers
inquiry
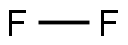
7782-41-4
1 suppliers
inquiry

373-91-1
11 suppliers
inquiry

927-84-4
9 suppliers
inquiry