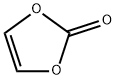
Vinylene carbonate synthesis
- Product Name:Vinylene carbonate
- CAS Number:872-36-6
- Molecular formula:C3H2O3
- Molecular Weight:86.05
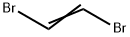
540-49-8
88 suppliers
$45.00/100mg
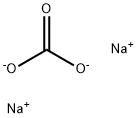
497-19-8
1383 suppliers
$14.00/1KG

872-36-6
388 suppliers
$5.00/5g
Yield:872-36-6 320 g
Reaction Conditions:
with potassium iodide in water at 40 - 92; for 12 h;Inert atmosphere;Reagent/catalyst;Temperature;
Steps:
1-2; 4; 1-2 Example 4 Preparation of vinylene carbonate
Under the protection of nitrogen, add 700 g of sodium carbonate and 3000 ml of deionized water to a 5000ml four-necked flask (with condenser), stir and raise the temperature to 40°C for complete dissolution, and then add 1000 g of 1,2-dibromoethylene.0.8 grams of potassium iodide,Polyethylene glycol 600 2 grams, continue to heat up to 92°C, keep the temperature for 12 hours, and cool down to room temperature. 500ml of chloroform was added to the reaction solution for extraction twice, the combined organic phase was dried by molecular sieve, filtered, and the solvent was removed by distillation under reduced pressure to obtain 444 grams of crude vinylene carbonate with a purity of 92.4% and a yield of 88.6%.Further, through high vacuum vacuum distillation (3mmHg) and crystallization (12-22°C), 320 grams of high-purity vinylene carbonate with a purity of 99.998% are obtained.
References:
CN111393403,2020,A Location in patent:Paragraph 0030-0033; 0036-0042
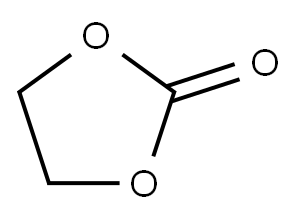
96-49-1
481 suppliers
$6.00/100g

872-36-6
388 suppliers
$5.00/5g

3967-54-2
219 suppliers
$21.00/25g

872-36-6
388 suppliers
$5.00/5g

5130-24-5
65 suppliers
$230.00/10ml

872-36-6
388 suppliers
$5.00/5g
