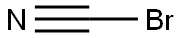Cyanogen bromide-Hazard and Toxicity
Sep 12,2019
Cyanogen bromide is a colorless to white crystalline solid with a penetrating odor. Cyanogen bromide is slightly soluble in water. Cyanogen bromide is gradually decomposed by water and very rapidly by acids to give off hydrogen bromide, a flammable and poisonous gas. Contamination with many materials can cause rapid decomposition of the material. Cyanogen bromide is toxic by inhalation of its vapors or by the hydrogen cyanide from decomposition or by ingestion. Toxic oxides of nitrogen are produced in fire involving Cyanogen bromide. Cyanogen bromide is used in gold extraction, to make other chemicals, and as a fumigant.

Toxicity Data
LCLO inhal (human)
92 ppm (398 mg/m3; 10 min)
LCLO inhal (mouse)
115 ppm (500 mg/m3; 10 min)
Major Hazards
High acute toxicity; severely irritating.
Toxicity
The acute toxicity of cyanogen bromide is high. Toxic effects are similar to but not as severe as those of hydrogen cyanide. Toxic symptoms may include cyanosis,nausea, dizziness, headache, lung irritation, chest pain, and pulmonary edema, which may be fatal.
Flammability and Explosibility
Cyanogen bromide is noncombustible. Impure material decomposes rapidly and can be explosive.
Reactivity and Incompatibility
Cyanogen bromide can react violently with large quantities of acid. It may decompose when exposed to heat, moist air, or water, producing toxic fumes of hydrogen cyanide and hydrogen bromide. Cyanogen bromide can polymerize violently on prolonged storage at ambient temperature.
Storage and Handling
Because of its high acute toxicity, cyanogen bromide should be handled using the "basic prudent practices" of Chapter 5.C, supplemented by the additional precautions for work with compounds of high toxicity (Chapter 5.D). In particular, work with BrCN should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Containers of cyanogen bromide should be kept tightly sealed and stored under nitrogen in a secondary container in a refrigerator.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If cyanogen bromide is ingested, obtain medical attention immediately. If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once.
In the event of a spill, sweep up cyanogen bromide, place in an appropriate container, and dispose of properly. Respiratory and appropriate impermeable protective gloves and clothing should be worn while conducting cleanup of this highly toxic substance.
Disposal
Excess cyanogen bromide and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
Uses
The main uses of cyanogen bromide are to immobilize proteins, fragment proteins by cleaving peptide bonds, and synthesize cyanamides and other molecules.
(1)Protein immobilization
Cyanogen bromide is often used to immobilize proteins by coupling them to reagents such as agarose for affinity chromatography.[6] Because of its simplicity and mild pH conditions, cyanogen bromide activation is the most common method for preparing affinity gels. Cyanogen bromide is also often used because it reacts with the hydroxyl groups on agarose to form cyanate esters and imidocarbonates. These groups are reacted with primary amines in order to couple the protein onto the agarose matrix, as shown in the figure. Because cyanate esters are more reactive than are cyclic imidocarbonates, the amine will react mostly with the ester, yielding isourea derivatives, and partially with the less reactive imidocarbonate, yielding substituted imidocarbonates.
The disadvantages of this approach include the toxicity of cyanogen bromide and its sensitivity to oxidation. Also, cyanogen bromide activation involves the attachment of a ligand to agarose by an isourea bond, which is positively charged at neutral pH and thus unstable. Consequently, isourea derivatives may act as weak anion exchangers.
(2)Protein cleavage
Cyanogen bromide hydrolyzes peptide bonds at the C-terminus of methionine residues. This reaction is used to reduce the size of polypeptide segments for identification and sequencing.
- Related articles
- Related Qustion
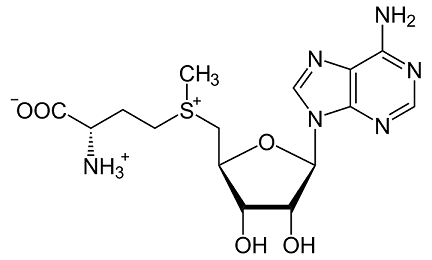
S-Adenosyl-L-methionine is a molecule that is formed naturally in the body. It can also be made in the laboratory. S-Adenosyl-L-methionine is involved in the formation, activation, or breakdown of other chemicals in the body, including horm....
Sep 11,2019Biochemical EngineeringDiazomethane has a molecular weight of 42.041 g/mol, and a monoisotopic mass of 42.022 g/mol, which is also its exact mass. Diazomethane has a musty odor, and it is shipped in the form of compressed gas.Diazomethane can be manufactured from....
Sep 12,2019Organic ChemistryCyanogen bromide
506-68-3You may like
Cyanogen bromide manufacturers
- Cyanogen bromide
-

- $3.00 / 1KG
- 2020-01-19
- CAS:506-68-3
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1kg,5kg,50kg