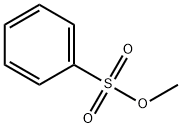
Methyl benzenesulfonate: Preparation, Reactions etc.
Mar 25,2020
Methyl benzenesulfonate is a sulfonate ester which acts as a potential genotoxic impurity in drug substances. Further, it exerts genotoxic effects in bacterial and mammalian cell systems. Incompatible with strong oxidizing agents, strong acids and strong bases [1].
The preparation of Methyl benzenesulfonate
Methyl benzenesulfonate is the effective methylating reagent of synthetic multiple organic intermediate, and industry is at present gone up and mainly adopted Phenylsulfonic acid and methyl alcohol to carry out after the esterification essence to steam, this technology starting material costliness, and reaction time is long, and product yield is low [2]. How to get Methyl benzenesulfonate with high yield, scientists have lots of researcher.
Liming Wang and his colleagues developed a method for preparing methyl benzenesulfonate (scheme 1), which comprises the following steps: adding benzene sulfonyl chloride into a reaction kettle, dropwisely adding a sodium methoxide methanol solution, keeping the temperature of 25-35 ℃ for 30 minutes, neutralizing to the pH value of 7-7.2, filtering, heating the filtrate to remove methanol, cooling to room temperature, and carrying out decolorization filtration to obtain a colorless transparent liquid methyl benzenesulfonatesulfonate, wherein the mol ratio of benzene sulfonyl chloride to sodium methoxide is 1:(1.2-1.4). The white crystal product can be prepared by changing the synthesis conditions; and thus, the invention is simple to operate, has the advantages of fewer steps, high yield and high practicality, can easily implement industrial production, and can generate high economic benefit and social benefit.
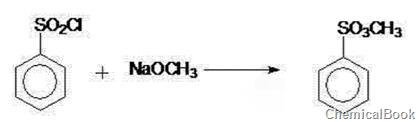
Scheme 1 Preparation of methyl benzenesulfonate
Reaction of Methyl benzenesulfonate
V. M. Nesterov and I. E. Chubova prepared theobromine from 3-methylxanthine, methyl benzenesulfonate, which has been used as the methylating agent [3]. To obtain theobromine, 3-methylxanthine is generally methylated with dimethyl sulfate in an aqueous ethanolic solution of caustic potash. However, if in the reaction the pH exceeds 8.0, the methylation of 3-methylxanthine leads to caffeine, which forms a by-product. The methylation of xanthine derivatives can also be carried out with methyl benzenesulfonate, but it takes place in a considerably more complicated way than with dimethyl sulfate. Since methyl benzenesulfonate, unlike dimethyl sulfate, undergoes practically no decomposition in neutral and weakly acidic media while methylation does not take place at a pH below 7.0-7.5, an excess of the methyl ester in the reaction mixture remains unchanged until the pH reaches the necessary figure. This considerably simplifies the performance of the methylation reaction. The detail process was listed as below.
Thirty grams of 3-methylxanthine was dissolved in 127 g of an 8% solution of caustic potash at 65℃ and 120 ml of methanol was added; then, at the same temperature, 30 ml of 95.5% methyl benzenesulfonate was added over 45 min; towards the end of the addition the pH became 7.5-8.0. The mixture was stirred for 20 min, the pH being maintained by the addition of an 8% solution of caustic potash, and then 19.5 ml of the ester were added over 15 min, the pH being kept within the same limits during an hour. Methylation was complete when 3-methylxanthine was no longer present in the reaction mixture, which was established by test with cobalt chloride. The mass was kept at 20-25℃ for 8 h, and then the theobromine was filtered off, washed with cold water, and mixed with 300 ml of water. A 20% solution of caustic potash was added to the suspension until the solid matter dissolved and then 2.5 g of activated carbon. After 30 min, the solution was filtered, and the theobromine was precipitated by the addition of 10% hydrochloric acid to pH 4.0, after which it was separated from the mother liquor and washed with water. Product was obtained with 75.4% yield (24.5 g).
Reference
[1] https://www.alfa.com/en/catalog/B24515/
[2] Liming Wang et. al., Method for preparing methyl benzenesulfonate, CN102351750A
[3] V. M. Nesterov and I. E. Chubova, Methylation of 3-methylxanthine with methyl benzenesulfonate, Novokuznets Chemical and Pharmaceutical Works. Transiatedfrom Khimiko-Farmatsevticheskii Zhurnal, No. 1, pp. 42-43, January, 1968. Original article submitted October 8, 1966. (https://link.springer.com/content/pdf/10.1007%2FBF00768118.pdf)
- Related articles
- Related Qustion
- Methyl benzenesulfonate: Complexes Synthesis and its Applications in Ethylene Polymerization Mar 27, 2024
Synthesis of methyl benzenesulfonate complexes showcasing their catalytic potential in ethylene polymerization and the scope for further enhancement through modifications.
- Methyl benzenesulfonate: properties and applications Sep 12, 2023
Methyl benzenesulfonate is a yellow liquid with excellent water solubility and stability. It is used in ethylene polymerization, methylation of 3-methylxanthine, and preparation of thin films.
Dibutyl phthalate is a colorless transparent oily liquid with a slight aromatic odor. It is insoluble in water, soluble in common organic solvents and hydrocarbons. It is obtained by using phthalic anhydride and n-butanol as raw materials.....
Mar 24,2020Catalyst and Auxiliary2-aminothiazole is a heterocyclic amine featuring a thiazole core and it can also be considered a cyclic isothiourea. 2-aminothiazole possesses an odor similar to pyridine and is soluble in water, alcohols and diethyl ether.....
Mar 25,2020Chemical ReagentsMethyl benzenesulfonate
80-18-2You may like
- How to synthesize Fluoroboric acid?
May 21, 2024
- Application of Polyphosphoric acid
Dec 31, 2021
- Introduction of Phosphoric acid
Nov 22, 2021
Methyl benzenesulfonate manufacturers
- Methyl benzenesulfonate
-

- $10.00 / 1KG
- 2024-11-05
- CAS:80-18-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Methyl benzenesulfonate
-

- $5.00 / 1KG
- 2024-10-11
- CAS:80-18-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000kg
- Methyl Benzenesulfonate, Methyl Benzene sulfonate
-

- $4200.00 / 1T
- 2024-08-28
- CAS:80-18-2
- Min. Order: 1KG
- Purity: 99% Min.
- Supply Ability: 200 tons per month