Production of Benzenesulfonic Acids and Their Derivatives
Mar 5,2021
The direct introduction of the sulfonic acid
group is one of the most important reactions in
industrial organic chemistry. Together with nitration
and chlorination, it belongs to the important
group of electrophilic aromatic substitution
reactions. It gives high yields under relatively
mild conditions and usually results in
well-defined benzene derivatives.
Direct Sulfonation
Aqueous sulfuric acid, at various concentrations from about 76% up to 100 %, is frequently used as the sulfonating agent. In industry 100 % sulfuric acid is often called monohydrate. Oleum is a solution of sulfur trioxide in sulfuric acid; for sulfonation it is generally used at SO3 concentrations of 20 or 65wt % because the solidification points at these two concentrations are minimal (0 and 2 ◦C, respectively). Other concentrations are less favorable with regard to storage and transportation because the solidification points are higher (for example, 45 % oleum solidifies already at 35 ◦C).
Sulfonation with sulfuric acid is a reversible
reaction (see also Figure 1):
ArH+H2SO4 → ArSO3H+H2O
It can be shifted optimally to the right if the
water of the reaction is bound or removed by
distillation. Higher temperatures usually shift
the equilibrium towards the right. However,
both methods increase sulfone formation.
The water of the reaction can also be bound
by adding thionyl chloride:
C6H6 +H2SO4 + SOCl2 −→ C6H5SO3H+SO2 + 2 HCl
For example, the sulfonation of benzene with sulfuric acid and thionyl chloride gives a mixture consisting of 96.3 % benzenesulfonic acid, 2.7 % sulfuric acid, and 1.6% diphenyl sulfone [29]. Similar yields are obtained when sulfonating toluene or chlorobenzene in this way.
No reaction water is formed in sulfonations
using chlorosulfuric acid or sulfur trioxide.
However, increased sulfone formation occurs.
As a sulfonating agent pure sulfur trioxide generally
reacts too violently and leads to extensive
side reactions including oxidation and sulfone
formation. Hence it is used in complexed form,
e.g., with pyridine, dioxane, trimethylamine, or
dimethylformamide. Many industrial processes
use sulfur trioxide gas as the sulfonating
agent, normally mixed with an inert gas such as
air, nitrogen, or carbon dioxide. Thus,
the sulfonating agent is diluted or evenly distributed
so that the heat of the reaction is quickly
dissipated (see also →Surfactants). In the liquid phase, sulfur dioxide or dichloromethane
is suitable as diluent. However, dichloromethane
reacts with sulfur trioxide at temperatures above
0 ◦C, and highly poisonous decomposition products
are formed, such as bis(chloromethyl) ether.
1,1-Dichloroethane also reacts with sulfur trioxide
at 80 ◦C; the decomposition products include
phosgene.
In stoichiometric amounts, chlorosulfuric
acid serves as a sulfonating agent according to ArH + ClSO3H −→ ArSO3H+ HCl
When using an excess of chlorosulfuric acid, sulfonyl chlorides are formed, see Section 1.9.1. The chlorosulfonation of toluene, for example, with chlorosulfuric acid at low temperature yields predominantly o-toluenesulfonyl chloride. This is the first step in the industrially important synthesis of saccharin (→Sweeteners).
Oxidation of Sulfur Compounds
Such sulfur compounds as thiophenols and diaryl disulfides can be oxidized with chlorine solution, permanganate, or nitric acid to form sulfonic acids. The process is industrially important wherever it is difficult or impossible to introduce the sulfonic acid group directly and the starting compounds are easily produced. An example of the use of halogens as oxidizing agents is given in.Diazo ReactionAryldiazonium halides are converted to aromatic sulfonic acids in glacial acetic acid containing SO2 in the presence of copper(I) chloride.
This reaction, which was first observed by L. Landsberg, permits the production of, for example, 1,2-benzenedisulfonic acid from orthoanilic acid with a yield of 68 %.

Sulfite Reaction
Aromatic halogen compounds can react with sulfite to form sulfonic acids if the halogen substituent is activated by nitro groups. For example, 1-chloro-2,4-dinitrobenzene reacts with sulfite to give 1,3-dinitro-4-benzenesulfonic acid. The reaction is catalyzed by copper ions. 2- Formylbenzenesulfonic acid and 2-sulfobenzoic acid are obtained similarly from the corresponding chlorine compounds.
The addition of hydrogensulfite to aromatic systems succeeds when their aromatic character is disturbed by the presence of certain substituents. The formation of 3-hydroxybenzenesulfonic acid from resorcinol and the sulfitation of m-dinitrobenzene with simultaneous reduction of a nitro group (Piria reaction) are examples:
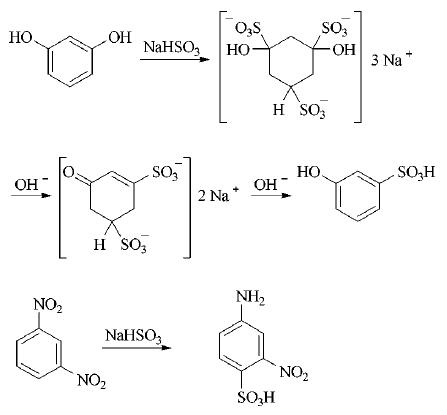
Hydrogensulfite addition, followed by the formation of a benzenesulfonic acid, gives satisfactory yields in special cases only and has therefore acquired little industrial importance so far.
- Related articles
- Related Qustion
Chlorhexidine digluconate, is a skin antiseptic in use in the health care setting with many potential applications for infection control. It is fungicidally active towards a wide range of Gram-positive and Gram-negative bacteria.....
Mar 5,2021Organic ChemistryHydroxychloroquine is a 4-aminoquinolone that is used to prevent and treat malaria, caused by parasitic protozoan from the Plasmodium genus.....
Mar 9,2021Antimicrobial agentBenzenesulfonic acid
98-11-3You may like
- Is benzoic acid polar or nonpolar?
Dec 21, 2023
- Hazards to the Environment and Humans of Ethyl Acetate
Nov 16, 2022
- Ethyl acetate - Uses, Properties, Safety etc.
Dec 29, 2021
Benzenesulfonic acid manufacturers
- Benzenesulfonic acid
-

- $8.80/ kg
- 2024-11-11
- CAS:98-11-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 tons
- Benzenesulfonic Acid
-

- $1.00 / 1kg
- 2024-11-05
- CAS:98-11-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
- Benzenesulfonic acid
-
- $0.00 / 1KG
- 2024-08-07
- CAS:98-11-3
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 10000KG






