The drug Benzimidazole
Sep 10,2024
Introduction
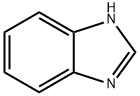
Benzimidazole, alternatively known as 1H-benzimidazole and 1,3-benzodiazole, consists of a benzene ring fused with a five-membered imidazole ring and is an important heterocyclic pharmacophore. Benzimidazole is regarded as a “privileged structure” in heterocyclic chemistry due to its association with various biological activities[1].

History
Back in the 1940s, benzimidazole was speculated to act similarly to purines to provide biological responses, and the first investigation on the biological activity of the benzimidazole nucleus was reported in 1944. The researchers' interest in the synthetic procedure of benzimidazole and its derivatives escalated when Brink et al. found that 5,6-dimethylbenzimidaozle was a degradation product of vitamin B12 and some of its derivatives also possessed vitamin B12 like activity. These early reports led researchers to explore benzimidazole nuclei for numerous activities[2].
Benefits
Through the course of many years of research, benzimidazole has emerged as an important heterocyclic system because of its existence in diverse biologically active compounds, such as antiparasitics, antimicrobials, antivirals, antifungals, anticonvulsants, antihypertensives, antihistaminics, analgesics, anti-inflammatory agents, anticancer, anticoagulants, and proton pump inhibitors. As a result of changing substituents around the core structure, many drugs of a wide variety of therapeutic lines have been developed, such as albendazole, mebendazole, thiabendazole as antihelmintics; enviradine as antiviral; carbendazim as fungicidal; omeprazole, lansoprazole, pantoprazole as proton pump inhibitors; candesartan cilexitil and telmisartan as antihypertensives, and astemizole as an antihistaminic agent. The high therapeutic potential of benzimidazole-related drugs has inspired medicinal chemists to carry out the synthesis of several novel chemotherapeutic agents containing benzimidazole moiety.
As a drug
Benzimidazole structures are classified under several ATC groups. The benzimidazole nucleus offers the basic possibility of substitution at seven different positions. Introduction of a small substituent into 2-position, and sometimes into 5-position, is characteristic of benzimidazole anthelmintics (ATC: Q09AA – veterinary anthelmintics; P02CA-human pharmaceuticals against helminths/flatworms, benzimidazole derivatives). Alternatively, bulky 2-substituents characterize the drugs used in the treatment of peptic ulcers and are sometimes referred to as proton pump inhibitors (ATC: A02BC – inhibitors of proton pump). Some drugs of this class also contain a small 5-substituent. Bulky 1- and 2-substituents are found in H1 antihistaminics (ATC: R06AX). Potential anti-virally active drugs were also studied, having substituents at various positions. The last class mentioned includes substances without pharmacological effect, which were only investigated from the point of view of dependence between structure and enzyme-modulating effect. In the first two ATC classes, all the substances in use (except for prodrugs of benzimidazole anthelmintics) contain the benzimidazole skeleton. Hence, it is assumed that this skeleton exerts the therapeutic effect. Benzimidazole anthelmintics are adopted worldwide, especially in veterinary medicine. They are indicated in the treatment and prevention of acute parasitic diseases. Benzimidazole anthelmintics are administered to species including dogs, cats, cattle, sheep, goats, pigs, horses, llamas, game, birds, poultry, reptiles, and man. The importance of benzimidazoles in veterinary medicine is documented by the wide spectrum of substances used and the variety of pharmaceuticals and dosage forms[3].
On the other hand, mebendazole, albendazole, and thiabendazole are only registered in human medicine. The drugs are commonly administered orally, while for parenteral administration, the more soluble derivatives, such as ricobendazole–albendazole sulphoxide, are available. The effective treatment of ruminants and horses can be reached with a single dose of benzimidazole drug, whereas repeated drug administration is required in men, pigs, dogs, and cats. Long-acting systems with stepwise release of drugs extending over as long as 140 days are also available. Of the proton pump inhibitors, only omeprazole is used in veterinary medicine. It is recommended for treating gastric and duodenal ulcerations, Zollingen–Ellison syndrome, and reflux oesophagitis in dogs and horses.
References
[1] Shejuti Rahman Brishty. “A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives.” Frontiers in Pharmacology (2021): 762807.
[2] Kamanna, Kantharaju. “Synthesis and Pharmacological Profile of Benzimidazoles.” Chemistry and Applications of Benzimidazole and its Derivatives 8 1 (2019): 0.
[3] J Velı́k . “Benzimidazole drugs and modulation of biotransformation enzymes.” Research in veterinary science 76 2 (2004): Pages 95-108.
- Related articles
- Related Qustion
- Benzimidazole: Chemical property, Primary Use, and biological activity Sep 3, 2024
Benzimidazole is a class of heterocyclic aromatic organic compounds which share a fundamental structural characteristic of six-membered benzene fused to the 4 and 5-position of the five-membered imidazole ring system.
- Properties, Synthesis and Reactivities of Benzimidazole Feb 11, 2022
Benzimidazole is a bicyclic planar, aromatic, 10π electron ring system, constituted by the fusion of a benzene ring with 4,5-positions of the imidazole ring. The nature of both the nitrogen atoms is different.
25-hydroxycholesterol can be generated by both enzymatic and nonenzymatic pathways. The enzyme responsible for 25-HC biogenesis is mainly the 25-hydroxylase.....
Sep 10,2024Biochemical EngineeringNalidixic acid has a bactericidal or bacteriostaic effect depending on the sensitivity of the microorganism and the concentration.....
Sep 10,2024DrugsBenzimidazole
51-17-2You may like
- Benzimidazole
-

- $36.00/ kg
- 2024-10-12
- CAS:51-17-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 tons
- Benzimidazole
-

- $0.00 / 1kg
- 2024-10-12
- CAS:51-17-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT
- Benzimidazole
-

- $6.00 / 1kg
- 2024-10-12
- CAS:51-17-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month