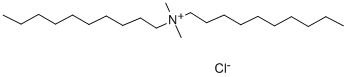
What is Didecyl dimethyl ammonium chloride?
Apr 12,2021
Didecyl dimethyl ammonium chloride is a clear, yellow liquid, yellowish powder or colorless crystals. It has a mushroom-like odor. It is moderately soluble in water. Didecyldimethylammonium chloride is a quaternary ammonium compound used as detergent/disinfectant in hospitals, as algicide in swimming pools, and as a fungicide and against termites in wood. This compound caused contact dermatitis in a hospital employee, also sensitive to glyoxal and bis-(aminopropyl)- laurylamine.

Application
In agriculture, Didecyldimethylammonium chloride is used as the Biocide, Fungicide, Bactericide, Herbicide, Algaecide, Algaecide, Bacteriocide, Fungistat, Microbiocide, Microbiostat disinfectant, Viricide, Tuberculocide, Molluscide, Insecticide, used as a general purpose disinfectant on hard, nonporous surfaces( as a sanitizer); used as a mildew preventative, wood preservative, and to kill algae, phytopathogenic fungi, phytopathogenic bacteria. It is an active ingredient in a large number of disinfectant products registered with USEPA and labeled with a claim to inactivate “avian influenza A” viruses on hard surfaces.
Didecyldimethylammonium chloride is used to disinfectant egg shells, milking equipment and udders, agricultural tools and vehicles. It is used as a sanitizer for swimming pools, and decorative ponds and fountains. It is a wood preservative. Didecyl dimethyl ammonium chloride is registered for these antimicrobial uses by the U.S. EPA Office of Pesticide Programs.
Exposure
Exposure to didecyl dimethyl ammonium chloride is most often from skin or eye contact, but can also include inhalation or ingestion. When didecyl dimethyl ammonium chloride is released to the environment, it will absorb to surfaces. It will move slowly or not at all in soil. It will not volatilize from soil or water surfaces. It will moderately build up in aquatic organisms. Microbes in the environment will break it down. Didecyl dimethyl ammonium chloride released to air will be in or on particles that eventually fall to the ground.
Toxicology & Risk
In mice this disinfectant was found to cause infertility and birth defects when combined with Alkyl (60% C14, 25% C12, 15% C16) dimethyl benzyl ammonium chloride (ADBAC). These studies contradict the older toxicology data set on quaternary ammonia compounds which was reviewed by the U.S. Environmental Protection Agency (U.S. EPA) and the EU Commission.
Didecyl dimethyl ammonium chloride is corrosive and can cause irreversible eye damage and skin burns. When using aerosol applications, swallowing or breathing in mists can be fatal. To prevent these short-term effects, the U.S. EPA Office of Pesticide Programs requires that personal protective equipment like chemical resistant gloves and apron be used, when applying certain products containing didecyl dimethyl ammonium chloride. The U.S. EPA Office of Pesticide Programs determined that didecyl dimethyl ammonium chloride is not likely to cause cancer, reproductive effects, or birth defects. This determination was based on results from studies with laboratory animals. The potential for didecyl dimethyl ammonium chloride to cause cancer in humans has not been assessed by the U.S. EPA IRIS program, the International Agency for Research on Cancer, or the U.S. National Toxicology Program 13th Report on Carcinogens. (SRC)
Contact allergens
Didecyldimethylammonium chloride (DDAC) is an antiseptic/disinfectant that is used in many biocidal applications. This quaternary ammonium compound is used as a detergent-disinfectant in hospitals, as an algaecide in swimming pools, as a fungicide, and against termites in wood. We observed severe contact dermatitis in a slaughterhouse worker using a liquid soap containing this product (personal observation). Immediate-type manifestations like urticaria and dyspnoea have been reported.
- Related articles
- Related Qustion
- Didecyl Dimethyl Ammonium Chloride: Antimicrobial Efficacy, Applications in Cleaning Products, and Safety Concerns Feb 19, 2024
Didecyl dimethyl ammonium chloride is an effective antimicrobial agent with health risks, necessitating careful handling and use in various cleaning products.
- Didecyl dimethyl ammonium chloride: pharmacokinetics, toxicology and applications Jul 7, 2023
Didecyl dimethyl ammonium chloride is a quaternary ammonium compound with antimicrobial properties. It finds applications in consumer disinfectants, industrial water treatment, and agricultural disinf
Lithium bis(trimethylsilyl)amide is a non-nucleophilic strong Br?nsted base, which is generally soluble in most of the nonpolar organic solvents. It is most commonly employed in organic reactions.....
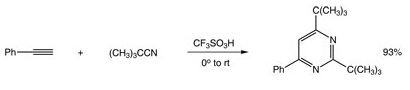
Apr 8,2021Organometallic compoundsTrifluoromethanesulfonic acid, also known as triflic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is often regarded as one of the strongest acids, and is one of a number of so-called "superacids".....
Apr 12,2021Organic reagentsDidecyl dimethyl ammonium chloride
7173-51-5You may like
Didecyl dimethyl ammonium chloride manufacturers
- Didecyl dimethyl ammonium chloride
-

- $0.00 / 200Kg/Drum
- 2025-04-18
- CAS:7173-51-5
- Min. Order: 1KG
- Purity: 80%
- Supply Ability: 2000mt
- Didecyl dimethyl ammonium chloride
-

- $999.00/ ton
- 2025-04-17
- CAS:7173-51-5
- Min. Order: 0.001ton
- Purity: 99%
- Supply Ability: 5000
- Didecyl dimethyl ammonium chloride
-

- $6.00 / 1kg
- 2025-04-17
- CAS:7173-51-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month