From early AD to mid-19 century, people mainly use the natural organic substance (such as animal and plant extracts) for qualitative analysis or quantitative analysis. From the second half of the 19th century to the 1920s, it had begun to appear of artificially synthetic organic reagent such as using potassium acetate xanthan for test of nickel, copper, and molybdenum; using morin for test of aluminum; using diazo coupling reaction for the detection of Nitrite; using α-β-nitroso naphthol for detection of cobalt; using dimethyglyoxime for nickel test. After the proposal of the special-effects group in the 1930s and the proposal of theoretical analysis of functional groups theory in 1950s, people had carried out large-scale screen of organic reagents in search of special-effects analysis groups for different ions and had successfully synthesized a lot of agents of practical value (such as copper reagents, new copper agent, cadmium reagents, beryllium reagent, thorium reagents, etc.). Before the 1950s, the complex compound, in analytic chemistry, is mainly used in the aspects of the precipitation reaction of a binary chelate for the qualitative detection, precipitate isolation and gravimetric separation and other aspects. In the early 1950s and 1960, it is mainly in the form of complexometric titration. From the beginning of the late 1960s, the main focus has been moved to the photometric analysis. Meanwhile, it has been also developed of chelate organic solvent extraction.
Q:What is Benzene used for?
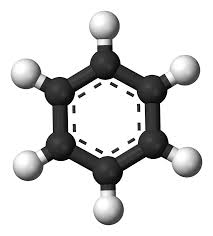
A:Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms.
Oct 30,2019 Organic reagentsQ:Health risk of Benzene
A:Benzene is a chemical that is released into the air from emissions from automobiles and burning coal and oil. It is also used in the manufacture of a wide range of industrial products, including chemi
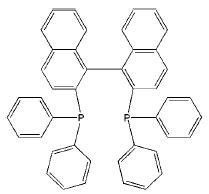
Oct 30,2019 Organic reagentsSynthesis and Preparation of (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
The crystal structure of (S)-(-)-BINAP] or C44H32P2, is enantiomorphous to the previously reported (R)-(+)-BINAP , with effectively no differences in the molecular geometry apart from being of opposit
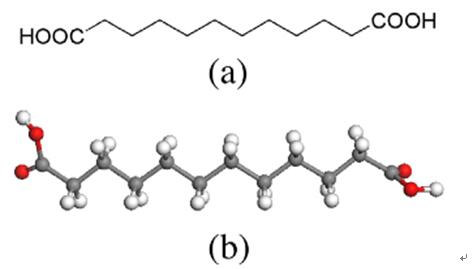
Oct 30,2019 Organic reagentsUses and Preparation of Dodecanedioic acid
Dodecanedioic acid (DDDA, C12H22O4, CAS registry No. 693-23-2) has achieved industrial importance in the manufacturing of polyamides, polyesters, lubricating oils and plastic.
Oct 23,2019 Organic reagentsThe properties and uses of 1,3-Propane sultone
1,3-Propane sultone is the organosulfur compound with the formula (CH2)3SO3. It is a cyclic sulfonate ester, a class of compounds called sultones. It is a readily melting colorless solid.
Sep 25,2019 Organic reagentsMethanol-Hazard and Toxicity
Methyl alcohol, also known as methanol or wood alcohol, is a clear, colorless, flammable liquid that is the simplest alcohol. World production of methanol is approximately 8.5 billion gallons annually
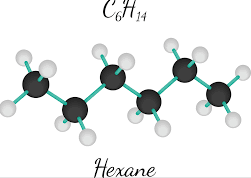
Sep 9,2019 Organic reagentsHexane-Hazard and Toxicity
n-Hexane is a highly flammable liquid, usually isolated from crude oil, and has extensive industrial applications as a solvent in adhesive bandage factories and other industries. It is highly toxic, t
Sep 6,2019 Organic reagentsFormaldehyde-Hazard and Toxicity
Formaldehyde, also called formic aldehyde or methyl aldehyde, has extensive application. For instance, it is used as a tissue preservative or organic chemical reagent. Thus, formaldehyde is very commo
Sep 6,2019 Organic reagentsEthidium bromide-Hazard and Toxicity
Ethidium bromide (abbreviated EB) is common fluorescent dyes of observing DNA under ultraviolet ray, it belongs intercalating dye because of its polycyclic structure, which enables it to be inserted b
Sep 6,2019 Organic reagents1,4-Dioxane-Hazard and Toxicity
1,4-dioxane is a clear liquid with ether-like odour. It is highly flammable and forms explosive peroxides in storage (rate of formation increased by heating, evaporation, or exposure to light). 1,4-Di
Sep 5,2019 Organic reagents