| Identification | Back Directory | [Name]
1,2,4,5-Tetrakis(4-carboxyphenyl)benzene | [CAS]
1078153-58-8 | [Synonyms]
H4TCPB
1,2,4,5-Tetrakis(4-carboxyphenyl)benzene
4,4',4'',4'''-benzene-1,2,4,5-tetrayl-tetrabenzoic acid
4',5'-Bis(4-carboxyphenyl)-[1,1':2',1''-terphenyl]-4,4''-dicarboxylic acid
1,2,4,5-Tetrakis(4-carboxyphenyl)benzene contains up to 6 wt. % water, >=98%
[1,1':2',1''-Terphenyl]-4,4''-dicarboxylic acid, 4',5'-bis(4-carboxyphenyl)-
1,2,4,5-Tetrakis(4-carboxyphenyl)benzene (This product is unavailable in the U.S.) | [Molecular Formula]
C34H22O8 | [MDL Number]
MFCD16621436 | [MOL File]
1078153-58-8.mol | [Molecular Weight]
558.53 |
| Hazard Information | Back Directory | [Uses]
| [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here. | [Synthesis]
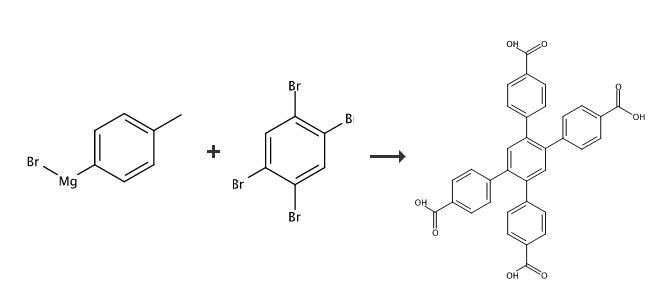
a) 100 ml of p-tolylmagnesium bromide (1M in THF, 100 mmol) was added under nitrogen to a flask containing 5g of (9.07 mmol). The mixture was stirred at nitrogen to a flask containing 5g of (9.07 mmol). The mixture was stirred at room temperature for 15 hours (gray suspension). The reaction was poured on ice then 50 ml of 6M HCl was added. The mixture was extracted with THF (3X 200ml). The organics were combined and the solvent was removed via rotary evaporation. The solid was then washed with hexanes and cold acetone. Isolated yield: 2.75g, 70% based on hexabromobenzene. yield: 2.75g, 70% 1H NMR (CDCl3): δ 2.32 (s, 12H), 7.04 (d, 8H), 7.12 (d, 8H), 7.45 (s, 2H). 13C NMR (CDCl3): δ 128.9, 130.0, 133.3, 136.4, 138.4, 139.5 ppm.(b) An amount of 2g from (a) was placed in a 100 ml teflon lined vessel. An amount of 24 ml of water and 6 ml of HNO3 were then added. The vessel was sealed and heated at 180 °C for 24 hrs. The resulting solid was collected by filtration and washed with THF/CHCl3 (7:3). Isolated yield: 1.85g, 75%. yield: 1.85g, 75% |
|
|