| Identification | Back Directory | [Name]
A77 1726 | [CAS]
108605-62-5 | [Synonyms]
Su 20
A77 1726
Aids013145
Aids-013145
Teriflunomide
TeriflunoMide, A77 1726
2-CYANO-3-OH-N-(4-TRIFLUOROMETHYLPHENYL) CROTONAMIDE
2-Cyano-3OH-N-(4-trifluoromethylphenyl) crotonamide-d4
N-(4-TRIFLUOROMETHYLPHENYL)-2-CYANO-3-HYDROXYCROTOAMIDE
N-(4-TRIFLUOROMETHYLPHENYL)-2-CYANO-3-HYDROXYCROTONAMIDE
n-(4-trifluoromethylphenyl)-2-cyano-2-hydroxycrotonamide
2-Cyano-3-hydroxy-N-(4-trifluoromethylphenyl)crotonamide
2-cyano-3-hydroxy-n-(4-(trifluoromethyl)phenyl)-2-butenamid
2-CYANO-3-HYDROXY-N-[4-(TRIFLUOROMETHYL)PHENYL]-2-BUTENAMIDE
2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide
2-Cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]but-2-enamide
(E)-2-Cyano-3-hydroxy-N-(4-trifluoromethylphenyl)-2-butenamide
2-Butenamide, 2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-
(Z)-2-cyano-3-hydroxy-N-(4-(trifluoroMethyl)phenyl)but-2-enaMide
IMp. A (EP) as Hydrochloride: 4-(TrifluoroMethyl)aniline Hydrochloride
2-Cyano-3-hydroxy-N-(4-(trifluoromethyl)phenyl)but-2-enamide,teriflunomide
(2Z)-2-[hydroxy-[[4-(trifluoromethyl)phenyl]amino]methylidene]-3-oxo-butanenitrile
Leflunomide Related Compound B (20 mg) (2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide)
Leflunomide Related Compound B (10 mg) (2-Cyano-3-hydroxy-N-(4'-trifluoromethylphenyl)-crotone amide)
N-(4-Trifluoromethylphenyl)-2-cyano-3-hydroxycrotonamide, 2-Cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-beuteamide, LEF-M | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C12H9F3N2O2 | [MDL Number]
MFCD00910058 | [MOL File]
108605-62-5.mol | [Molecular Weight]
270.21 |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
229-232°C | [Boiling point ]
410.8±45.0 °C(Predicted) | [density ]
1.424±0.06 g/cm3(Predicted) | [storage temp. ]
+2C to +8C | [solubility ]
Soluble in DMSO (up to 30 mg/ml), or in Ethanol (up to 5 mg/ml with warming). | [form ]
neat | [pka]
5.20±0.50(Predicted) | [color ]
White | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Uses]
The active metabolite of Leflunomide, a potent disease-modifying antirheumatic drug used in the treatment of rheumatoid arthritis. LEF-M interferes with dendritic cell function | [Description]
Teriflunomide (Aubagio®), also known as A77 1726, is an immunosupressant marketed by Sanofi for
the teatment of multiple sclerosis (MS). Teriflunomide is the active metabolite of leflunomide, used
for treatment of patients diagnosed with rheumatoid arthritis, and therefore simultaneously can be used
as a treatment for rheumatoid arthritis.Teriflunomide acts as an inhibitor of the mitochondrial
enzyme dihydrorotate dehydrogenase, inhibiting pyrimidine formation, and resulting in reduced
B and T cell proliferation. | [Definition]
ChEBI: Teriflunomide is an enamide obtained by formal condensation of the carboxy group of (2Z)-2-cyano-3-hydroxybut-2-enoic acid with the anilino group of 4-(trifluoromethyl)aniline. Used for the treatment of relapsing forms of multiple sclerosis and rheumatoid arthritis. It has a role as an EC 1.3.98.1 [dihydroorotate oxidase (fumarate)] inhibitor, a tyrosine kinase inhibitor, a hepatotoxic agent, a drug metabolite and a non-steroidal anti-inflammatory drug. It is a nitrile, an enol, an aromatic amide, an enamide, a member of (trifluoromethyl)benzenes and a secondary carboxamide. | [Clinical Use]
Immunomodulating agent:
Treatment of relapsing remitting multiple sclerosis | [Synthesis]
Numerous syntheses of teriflunomide have been developed to date, most relying on the use of 4-
trifluoromethyl aniline (167). The current optimized method for scale-up synthesis of teriflunomide,
developed by Keshav and coworkers, begins with reaction of commercial 4-trifluoromethyl aniline 167
and ethylacetoacetate (168) in refluxing xylenes, providing acetoamidate 169 in 51% yield . The resulting acetoamidate 169 was then treated with H2O2, KBr, and concentrated HCl at room
temperature, providing bromide 170 in 67% yield. Bromide 170 was reacted with NaCN in DMSO,
generating teriflunomide (XXVI) in 85% yield.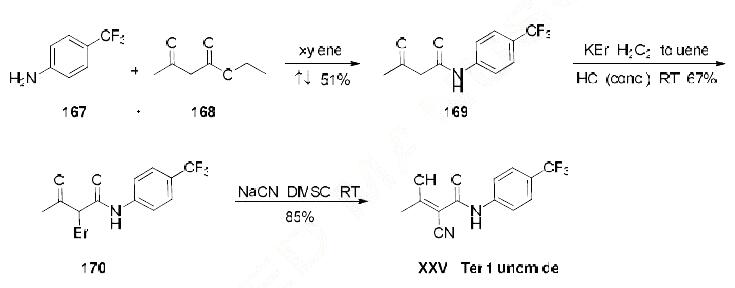
| [Drug interactions]
Potentially hazardous interactions with other drugs
Lipid-lowering agents: effect significantly reduced by
colestyramine - avoid; concentration of rosuvastatin
increased - consider reducing rosuvastatin dose.
Live vaccines: risk of generalised infections - avoid. | [Metabolism]
Teriflunomide is the active metabolite of leflunomide.
It is moderately metabolised and teriflunomide is
the only component detected in plasma. The main
biotransformation pathway is hydrolysis with oxidation
being a minor pathway. Secondary pathways involve
oxidation, N-acetylation and sulfate conjugation.
Teriflunomide is excreted in the gastrointestinal tract
mainly through the bile as unchanged drug and most
likely by direct secretion. | [storage]
Store at +4°C | [References]
1) Manna et al. (1999), Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression; J. Immunol., 162 2095
2) Davis et al. (1996), immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase; Biochemistry, 35 1270
3) Seah et al. (2008), Oxidative bioactivation and toxicity of leflunomide in immortalized human hepatocytes and kinetics of the non-enzymatic conversion to its major metabolite, A77 1726; Drug Metab. Lett., 2 153 |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
22 | [HS Code ]
2926900005 | [Toxicity]
mouse,LD50,intraperitoneal,100mg/kg (100mg/kg),United States Patent Document. Vol. #5494911, |
|
|