| Identification | Back Directory | [Name]
Dabrafenib Mesylate(GSK-2118436B) | [CAS]
1195768-06-9 | [Synonyms]
GSK 2118436B
Dapafini mesylate
abrafenibmesylate
Dabrfenib Mesylate
GSK2118436 Mesylate
Dabrafenib Mesylate
GSK 2118436 Mesylate
GSK-2118436 Mesylate
Dabrafenib Mesylate (API)
Debrafenib Mesylate (API)
Dalafenib methanesulfonate
Dabrafenib, Methanesulfonate Salt
GSK 2118436 methanesulfonate salt
Dabrafenib Mesylate (GSK-2118436)
Dabrafenib Mesylate(GSK-2118436B)
Dabrafenib Mesylate (GSK2118436 Mesylate
GSK2118436 Ms salt, Dabrafenib Ms salt, GSK2118436A Ms salt
N-(3-(5-(2-Aminopyrimidin-4-yl)-2-(tert-butyl)thiazol-4-yl)-2-fluorophenyl)-2,6-difluorobenzen
DABRAFENIB MESYLATE (GSK 2118436B);GSK-2118436 MESYLATE;GSK2118436 MESYLATE;GSK 2118436 MESYLATE
N-(3-(5-(2-Aminopyrimidin-4-yl)-2-(tert-butyl)thiazol-4-yl)-2-fluorophenyl)-2,6-difluorobenzenesulfonamide methanesulfonate
N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluoro-benzenesulfonamide methanesulfonate
N-{3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide methanesulfonic acid
Benzenesulfonamide, N-[3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluoro-, methanesulfonate (1:1) | [EINECS(EC#)]
689-167-4 | [Molecular Formula]
C24H24F3N5O5S3 | [MDL Number]
MFCD20922872 | [MOL File]
1195768-06-9.mol | [Molecular Weight]
615.668 |
| Chemical Properties | Back Directory | [Melting point ]
>234oC (dec.) | [storage temp. ]
Hygroscopic, -20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly, Heated), Methanol (Slightly) | [form ]
White solid. | [color ]
White to Off-White | [Stability:]
Hygroscopic | [InChIKey]
YKGMKSIHIVVYKY-UHFFFAOYSA-N | [SMILES]
S(O)(=O)(=O)C.FC1C(=CC=CC=1C1N=C(C(C)(C)C)SC=1C1C=CN=C(N)N=1)NS(C1C(=CC=CC=1F)F)(=O)=O |
| Questions And Answer | Back Directory | [Description]
Dabrafenib Mesylate(1195768-06-9) is a salt form of Dabrafenib. Dabrafenib is an inhibitor of certain mutated forms of BRAF kinase, several of which may be associated with stimulating tumour growth (e.g. the BRAF V600E mutation), and Dabrafenib is also an inhibitor of BRAF V600-mutation positive cancer cell growth, both in vitro and in vivo.
|
| Hazard Information | Back Directory | [Uses]
Dabrafenib methanesulfonate salt is a selective BRAF V600E inhibitor. Dabrafenib methanesulfonate salt binds to Raf family kinases and inhibits their activity. | [Definition]
ChEBI: A methanesulfonate (mesylate) salt prepared from equimolar amounts of dabrafenib and methanesulfonic acid. Used for treatment of metastatic melanoma. | [Synthesis]
Commercially available fluoroaniline 40 was first converted to
sulfonamide 42 in 91% yield by treatment with 2,5-difluorobenzenesulfonyl
chloride (41) in the presence of pyridine. Next, deprotonation
of 2-chloro-4-methylpyrimidine (43) with lithium
bis(trimethylsilyl)amide (LHMDS) followed by addition to ester
42 afforded chloropyrimidine 44 in 72% yield. Bromination followed
by thiazole formation through the use of 2,2-dimethylpropanethioamide
gave the penultimate target 45 in 80% over
two steps. Chloropyrimidine 45 was subjected to SNAr conditions with ammonium hydroxide to furnish the aminopyrimidine in 88%
yield, and this was followed by exposure to methanesulfonic acid
to afford dabrafenib mesylate (VI) in 85% yield.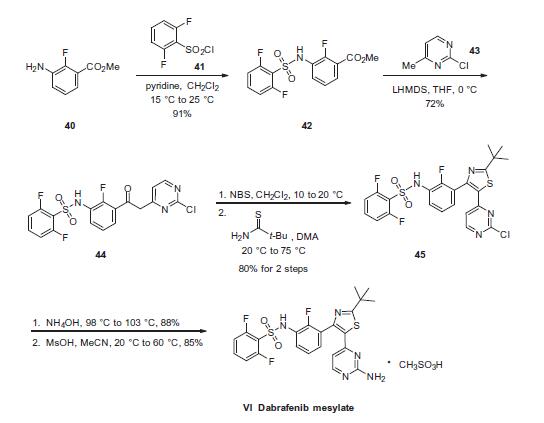
| [target]
Raf | [storage]
Store at -20°C | [References]
[1]namba h, nakashima m, hayashi t, hayashida n, maeda s, rogounovitch ti, ohtsuru a, saenko va, kanematsu t, yamashita s. clinical implication of hot spot braf mutation, v599e, in papillary thyroid cancers. j. clin. endocrinol. metab. 2003; 88 (9): 4393–7.
[2]tan yh, liu y, eu kw, ang pw, li wq, salto-tellez m, iacopetta b, soong r. detection of braf v600e mutation by pyrosequencing. pathology 2008; 40 (3): 295–8.
[3]davies h, bignell gr, cox c, et al. mutations of the braf gene in human cancer. nature. 2002; 417: 949-954.
[4]ma xh, piao sf, dey s, mcafee q, karakousis g, villanueva j, hart ls, levi s, hu j, zhang g, lazova r, klump v, pawelek jm, xu x, xu w, schuchter lm, davies ma, herlyn m, winkler j, koumenis c, amaravadi rk. targeting er stress-induced autophagy overcomes braf inhibitor resistance in melanoma. j clin invest. 2014; 124(3): 1406-17. |
|
|