| Identification | Back Directory | [Name]
Chloro(1,5-cyclooctadiene)iridium(I) dimer | [CAS]
12112-67-3 | [Synonyms]
[Ir(1,5-cod)Cl]2
IRIDIUM COD CHLORIDE
IRIDIUM I CYCLOOCTADIENE CHLORIDE
IRIDIUM CHLORO-1,5-CYCLOOCTADIENE
chloro(1,5-cyclooctadiene)iridium(i)
1,5-octadiene iridiuM chloride diMer
BIS(1,5-CYCLOOCTADIENE)DIIRIDIUM(I) DIC&
Chloro-(1,5-cyclooctadiene)-iridium dimer
IRIDIUM(I) CYCLOOCTADIENE CHLORIDE, diMer
Chloro(1,5-cyclooctadiene)iridium(Ⅰ) dimer
Chloro(1,5-cyclooctadiene)iridium(I)dimere
CHLORO(1,5-CYCLOOCTADIENE)IRIDIUM(I) DIMER
CHLORO(1,5-CYCLOOCTADIENE)IRIDATE (I) DIMER
Chloro-1,5-cyclooctadieneiridium(I)dimer,99%
1,5-CYCLOOCTADIENE-IRIDIUM(I) CHLORIDE DIMER
BIS(1,5-CYCLOOCTADIENE)DIIRIDIUM(I) DICHLORIDE
BIS(1,5-CYCLOOCTADIEN)DIIRIDIUM(I)-DICHL ORIDE
Chloro(1,5-cyclooctadiene)iridium(I) dimer ,98%
"Di-chloro bis(cycloocta-1,5-dienyl)iridium(I)"
Chloro(1,5-cyclooctadiene)iridiuM(I) diMer 250MG
Chloro(1,5-cyclooctadiene)iridium(I), dimer,99.9%
Bis(1,5-cyclooctadiene)diiridiuM(I) dichloride 97%
CHLORO(1,5-CYCLOOCTADIENE)IRIDIUM(I) DIMER (57%IR)
IRIDIUM(I) CHLORIDE 1,5-CYCLOOCTADIENE COMPLEX DIMER
Chloro(1,5-cyclooctadiene)iridium(I)dimer dec. 190
Chloro(1,5-cyclooctadiene)iridium(I) dimer, Ir 57.2%
DI-MU-CHLOROBIS[(ETA-CYCLOOCTA-1,5-DIENE)IRIDIUM (I)]
Chloro(1,5-cyclooctadiene)iridiuM(I) diMer,[Ir(COD)Cl]2
IridiuM, di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]di-
di-mu-chlorobis[(1,2,5,6-eta)cycloocta-1,5-diene]diiridium
DI-MU-CHLORO-BIS[(1,2,5,6-ETA)-1,5-CYCLOOCTADIENE]DIIRIDIUM
Chloro(1,5-cyclooctadiene)iridiuM(I) diMer,98% [Ir(COD)Cl]2
Bis(1,5-cyclooctadiene)diiridium(I) dichloride,1,5-Cyclooctadiene-iridium(I) chloride dimer, Chloro(1,5-cyclooctadiene)iridium(I) dimer, Di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]diiridium, Iridiu
1,5-Cyclooctadiene-iridium(I) chloride dimer, Chloro(1,5-cyclooctadiene)iridium(I) dimer, Iridium(I) chloride 1,5-cyclooctadiene complex dimer, Di-μ-chlorobis[(1,2,5,6-η:)-1,5-cyclooctadiene]diiridium | [EINECS(EC#)]
235-170-1 | [Molecular Formula]
C16H24Cl2Ir2 | [MDL Number]
MFCD00012414 | [MOL File]
12112-67-3.mol | [Molecular Weight]
671.7 |
| Chemical Properties | Back Directory | [Appearance]
red-orange solid | [Melting point ]
205 °C (dec.)(lit.)
| [storage temp. ]
2-8°C
| [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Powder | [color ]
red to orange | [Water Solubility ]
insoluble | [Hydrolytic Sensitivity]
7: reacts slowly with moisture/water | [InChIKey]
XHOSESNLNGITPM-XRGHXPOKSA-L |
| Hazard Information | Back Directory | [Chemical Properties]
red-orange solid | [Uses]
Chloro(1,5-cyclooctadiene)iridium(I) dimer is widely used as a precursor to other iridium complexes, which finds application in homogeneous catalysis like carbonylation, hydrosilylation, hydrofomylation, asymmetric allylic substitutions, metathesis and chiral catalysis reactions. It is involved in the preparation of Crabtree's catalyst, which is used for hydrogenation and hydrogen-transfer reactions. | [reaction suitability]
core: iridium
reaction type: C-H Activation
reagent type: catalyst | [Synthesis]
A solution of 6 mL of 1,5-cyclooctadiene in 35 mL of ethanol and 20 mL of water is added to 2.0 g of IrCl3.3H2O in a round-bottomed flask. The mixture is refluxed under nitrogen for 24 h when an orange-red product precipitates from the solution. The mixture is cooled to rt, and Di-μ-chlorobis(1,5-cyclooctadiene)diiridium(I) (Chloro(1,5-cyclooctadiene)iridium(I) dimer) is collected by filtration, washed with cold methanol, and dried in vacuo at 25 °C for 8 h.
|
| Questions And Answer | Back Directory | [Reactions]
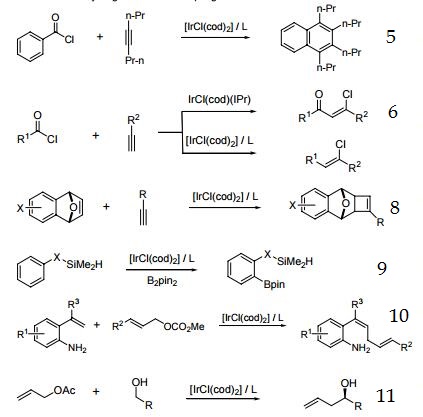
1. Precursor to catalysts for the asymmetric hydrogenation of tri- and tetrasubstituted olefins.
2. Precursor to catalyst for enantioselective reduction of imines.
3. Precursor to catalyst for allylic alkylation.
4. Precursor to catalyst for allylic amination and etherification.
5. Precursor to catalyst for the reaction of aroyl chlorides with internal alkynes to produce substituted naphthalenes and anthracenes.
6. Ir-catalyzed addition of acid chlorides to terminal alkynes.
7. Intramolecular hydroamination of unactivated alkenes with secondary alkyl- and arylamines.
8. Enantioselective [2+2] cycloaddition.
9. Silyl-directed, Ir-catalyzed ortho-borylation of arenes.
10. Ir-catalyzed cross-coupling of styrene derivatives with allylic carbonates.
11. Transfer hydrogenative C-C coupling
|
|
|