| Identification | Back Directory | [Name]
BUTABARBITAL | [CAS]
125-40-6 | [Synonyms]
Nilox
Butatab
Butatal
Butisol
Butrate
Secumal
Buticaps
Medarsed
Butabarb
Secbutab
NSC 27517
Unicelles
butabaritone
BUTABARBITAL
Butabarbitone
Secbubarbital
Secbutabarbital
Secbutobarbital
Secbutobarbitone
secbubarbitalsodium
component of Pyridium plus
Butabarbital CIII (200 mg)
Butabarbital solution
5-sec-butyl-5-ethylmalonylurea
5-sec-Butyl-5-ethylmalonyl urea
5-sec-butyl-5-ethyl-barbituricaci
5-sec-butyl-5-ethylbarbituricacid
5-sec-Butyl-5-Ethylbarbituric acid
5-ethyl-5-sec-butyl-barbituric acid
Methanol (test Butabarbital,1.0mg/mL)
5-ethyl-5-(1-methylpropyl)barbiturate
Barbituric acid, 5-sec-butyl-5-ethyl-
5-ethyl-5-(1-methylpropyl)barbituricacid
5-Ethyl-5-(1-methylpropyl)barbituric acid
5-butan-2-yl-5-ethyl-1,3-diazinane-2,4,6-trione
5-Sec-butyl-5-ethyl-2,4,6(1H,3H,5H)-pyrimidinetrione
6(1h,3h,5h)-pyrimidinetrione,5-ethyl-5-(1-methylpropyl)-4
5-Ethyl-5-(1-methylpropyl)-2,4,6(1H,3H,5H)-pyrimidinetrione
2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethyl-5-(1-methylpropyl)- | [EINECS(EC#)]
204-738-6 | [Molecular Formula]
C10H16N2O3 | [MDL Number]
MFCD00057560 | [MOL File]
125-40-6.mol | [Molecular Weight]
212.25 |
| Hazard Information | Back Directory | [Uses]
Controlled substance (depressant). Sedative, hypnotic. | [Description]
Butabarbital (CRM) (Item No. 20088) is a certified reference material categorized as a barbiturate. It has a high abuse potential and increases risk of overdose morbidity and mortality in recreational drug users. Butabarbital is regulated as a Schedule III compound in the United States. Butabarbital (CRM) (Item No. 20088) is provided as a DEA exempt preparation. This product is intended for research and forensic applications. | [Definition]
ChEBI:Butabarbital is a member of barbiturates. | [Brand name]
Butabarb (Alpharma);
Butalan (Lannett); Buticaps (Medpointe); Butisol Sodium
(Medpointe); Sarisol (Halsey). | [Synthesis]
Butabarbital, 5-ethyl-5-isobutylbarbituric acid (4.1.11), is also synthesized
in an analogous manner by condensation of |á-ethyl-|á-isobutylmalonic ester with urea [9].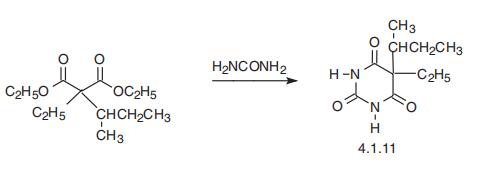
|
|