| Identification | Back Directory | [Name]
4-(Bromomethyl)-7-(diethylamino)coumarin | [CAS]
1256259-14-9 | [Synonyms]
4-(Bromomethyl)-7-(diethylamino)coumarin
2H-1-Benzopyran-2-one, 4-(bromomethyl)-7-(diethylamino)- | [Molecular Formula]
C14H16BrNO2 | [MOL File]
1256259-14-9.mol | [Molecular Weight]
310.19 |
| Chemical Properties | Back Directory | [Boiling point ]
435.8±45.0 °C(Predicted) | [density ]
1.407±0.06 g/cm3(Predicted) | [form ]
powder to crystal | [pka]
3.21±0.20(Predicted) | [color ]
Light yellow to Brown | [InChI]
InChI=1S/C14H16BrNO2/c1-3-16(4-2)11-5-6-12-10(9-15)7-14(17)18-13(12)8-11/h5-8H,3-4,9H2,1-2H3 | [InChIKey]
JPBOBQNHSXLIBI-UHFFFAOYSA-N | [SMILES]
C1(=O)OC2=CC(N(CC)CC)=CC=C2C(CBr)=C1 |
| Hazard Information | Back Directory | [Research]
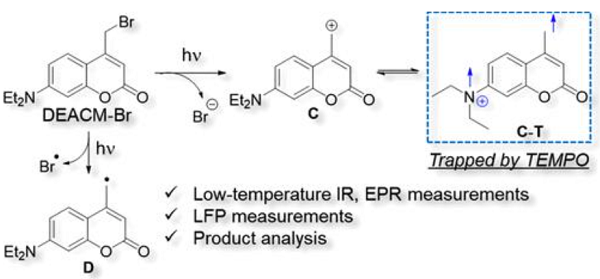
7-Diethylamino-4-methyl coumarin (DEACM) derivatives are widely used as photolabile protecting groups. The photolysis of 4-(Bromomethyl)-7-(diethylamino)coumarin (DEACM-Br) with Br as the leaving group was investigated by Takanoet al. The main reaction path was found to be the generation of radical D via homolytic C–Br bond cleavage. Interestingly, products derived from C-T, the triplet state of the carbocation intermediate (i.e., 7-(diethylamino)-4-methyl cation (C)), were identified, thereby confirming the existence of C-T for the first time[1].
| [References]
[1] Ma-aya Takano."Photoreaction of 4-(Bromomethyl)-7-(diethylamino)coumarin: Generation of a Radical and Cation TripletDiradical during the C-Br Bond Cleavage." Organic Letters (2022).
|
|
| Company Name: |
TCI Europe
|
| Tel: |
320-37350700 |
| Website: |
https://www.tcichemicals.com/de/de/index.html |
| Company Name: |
TCI AMERICA
|
| Tel: |
800-4238616 |
| Website: |
https://www.tcichemicals.com/en/us/index.html |
| Company Name: |
Alfa Chemistry
|
| Tel: |
1-516-6625404 |
| Website: |
https://www.alfa-chemistry.com |
| Company Name: |
TCI Chemicals
|
| Tel: |
021-67121386, 800-988-0390 |
| Website: |
www.tcichemicals.com |
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|