| Identification | Back Directory | [Name]
2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol | [CAS]
128607-22-7 | [Synonyms]
CS-1039
Fc-1271a
Ospemifene
Ccris 9205
Ospemifene >
Unii-B0p231ilbk
2-(4-(4-chloro-1
Ospemifene FC-1271a
2-diphenyl-but-1-enyl)phenoxy)ethanol
2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol
Z-2-[4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy]ethanol
2-(p-((Z)-4-Chloro-1,2-diphenyl-1-butenyl)phenoxy)ethanol
2-[4-[(Z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]ethanol
2-[4-[(1Z)-4-chloro-1,2-diphenyl-1-buten-1-yl]phenoxy]-ethanol
Ethanol,2-[4-[(1Z)-4-chloro-1,2-diphenyl-1-buten-1-yl]phenoxy]-
2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol USP/EP/BP | [EINECS(EC#)]
664-452-6 | [Molecular Formula]
C24H23ClO2 | [MDL Number]
MFCD00871890 | [MOL File]
128607-22-7.mol | [Molecular Weight]
378.897 |
| Chemical Properties | Back Directory | [Boiling point ]
544.6±50.0 °C(Predicted) | [density ]
1.166±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
DMSO: soluble20mg/mL, clear | [form ]
powder | [pka]
14.26±0.10(Predicted) | [color ]
white to beige | [InChIKey]
LUMKNAVTFCDUIE-VHXPQNKSSA-N | [CAS DataBase Reference]
128607-22-7 |
| Hazard Information | Back Directory | [Description]
In February 2013, the US FDA approved ospemifene (also referred to as FC1271a), for the treatment ofmoderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy (VVA), due tomenopause. It is estimated that there are 150 million postmenopausal women worldwide with 40–70% suffering from VVA. Ospemifene is a selective estrogen receptor (ER) modulator (SERM) and the first nonhormonal, nonestrogen for the treatment of moderate to severe dyspareunia in women with menopausal VVA. It binds to ERα (IC50~800 nM) and ERβ (IC50~1600 nM) with tissue-specific estrogenic agonist/antagonist effects. Treatment with ospemifene increases the thickness of the vaginal tissue thereby decreasing fragility of the tissue and reducing potential for pain during sexual intercourse. | [Originator]
Tess Diagnostics and
Pharmaceuticals/Hormos
Medical/QuatRx (Finland) | [Uses]
Treatment of vaginal atrophy, osteoporosis, and vasomotor symptoms. | [Definition]
ChEBI: An organochlorine compound that is a selective estrogen receptor modulator; used for treatment of dyspareunia. | [Brand name]
Osphena | [Clinical Use]
Ospemifene is a SERM that is currently in Phase II/III clinical trials for the treatment of postmenopausal
osteoporosis and urogenital atrophy. It is a known metabolite of toremifene, a triphenylethylene
derivative used to treat breast cancer.Ospemifene has been shown to have beneficial effects on the bone
without significant estrogen-related side effects. The beneficial effect observed on bone stems from this
agent's ability to increase osteoblast proliferation and, as a result, to enhance bone mineralization as well as
bone formation. Unlike tamoxifen, ospemifene does not induce osteocyte apoptosis. | [Synthesis]
The drug can be synthesized succinctly in two steps. First, alkylation
of commercially available 4-hydroxybenzophenone (130)
with ethylene carbonate and catalytic sodium iodide in refluxing
toluene provided benzophenone 131 in 94% yield. This was followed
by a McMurry coupling involving benzophenone 131 with
chloropropiophenone 132 in the presence of zinc powder and titanium
tetrachloride in 2-methyltetrahydrofuran. This reaction gave
rise to a mixture of triphenylethylenes directly as a 5.5:1 ratio of Z
to E isomers which could be separated by crystallization in aqueous
methanol to give a mixture of olefins, 98% of which was comprised
of the desired Z-isomer corresponding to ospemifene (XVII).
The product purity was further improved by recrystallization to
give 99.9% of the Z-isomer in 46% yield from 131. Thus, ospemifene
was synthesized in two steps and 43% overall yield.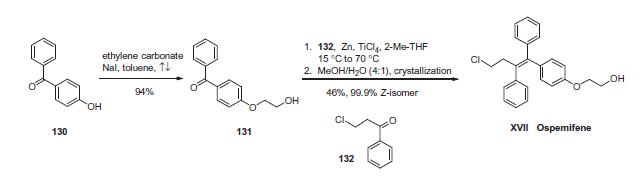
| [storage]
Store at -20°C |
|
|