| Identification | Back Directory | [Name]
Cyclomethycaine | [CAS]
139-62-8 | [Synonyms]
Topocaine
Surfacaine
Surfathesin
Cyclomethycaine
Cyclomethycaine USP/EP/BP
3-(2-methylpiperidin-1-yl)propyl 4-cyclohexyloxybenzoate
4-(Cyclohexyloxy)benzoic acid 3-(2-methyl-1-piperidinyl)propyl
4-(cyclohexoxy)benzoic acid 3-(2-methyl-1-piperidyl)propyl ester
Benzoic acid,4-(cyclohexyloxy)-, 3-(2-methyl-1-piperidinyl)propyl ester | [Molecular Formula]
C22H33NO3 | [MOL File]
139-62-8.mol | [Molecular Weight]
359.507 |
| Hazard Information | Back Directory | [Originator]
Surfacaine,Lilly,US,1948 | [Uses]
Cyclomethycaine is also used in topical anesthesia on the skin or mucous membranes for cuts,
bites, and also for urological examinations. A common synonym of this drug is surfacaine. | [Definition]
ChEBI: Cyclomethycaine is a benzoate ester. | [Manufacturing Process]
7.4 g of sodium are dissolved in 250 cc of isoamyl alcohol, 53 g of ethyl p�hydroxybenzoate are added and the mixture is heated to refluxing
temperature for about 15 minutes. To the cooled mixture, 65 g of cyclohexyl
bromide are added and the mixture is refluxed for about 3 hours. The isoamyl
alcohol is removed by evaporation in vacuo and the residue is extracted with
10% aqueous sodium hydroxide solution to remove the unreacted ethyl p�hydroxybenzoate.
The alkali-insoluble residue comprising ethyl p-cyclohexyloxybenzoate is
hydrolyzed by refluxing with 10% sodium hydroxide solution for about 3
hours. The alkaline reaction mixture is acidified with hydrochloric acid
whereupon p-cyclohexyloxybenzoic acid precipitates. The precipitate is
separated by filtration, washed with water and dried. It melts at about 178°
to 180°C. Yield: about 7%.
62 g of p-cyclohexyloxybenozic acid and 49.5g of 3-(2'-methylpiperidino)-
propyl chloride are dissolved in 300 cc of dry isopropanol and the mixture
refluxed for about 12 hours. About half of the isopropanol is then distilled off
and the residual solution cooled to about 0°C. 3(2'-methylpiperidino)-propyl
p-cyclohexyloxybenzoate hydrochloride precipitates as a white crystalline
compound. It is filtered off, washed once with ether and recrystallized from
isopropanol.
3(2'-Methylpiperidino)-propyl p-cyclohexyloxybenzoate hydrochloride thus
prepared melted at about 178° to 180°C. Analysis showed the presence of
8.88% chlorine as compared with the calculated value of 8.96%. | [Brand name]
Surfacaine (Lilly). | [Therapeutic Function]
Local anesthetic | [Synthesis]
Cyclomethycaine, the ethyl ester of 3-(2-methylpiperidino)propyl-o�cyclohexyloxybenzoic acid (2.3.4), is synthesized according to the figure below. Alkylation
of 2-methylpiperidine with 3-chlorpropanol-1 gives 3-(2-methylpiperidino)propanol-1
(2.3.2), whose hydroxyl group is substituted by chlorine using thionyl chloride. The result�ing 3-(2-methylpiperidino)propylchloride-1 (2.3.3) is further reacted with 4-cyclohexyl�oxybenzoic acid, which gives cyclomethycaine [27,28].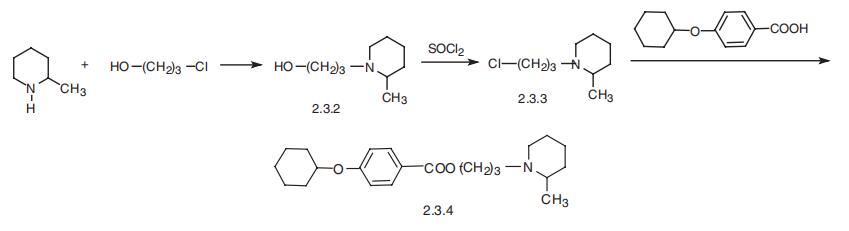
|
|
| Company Name: |
CHEMICAL LAND21
|
| Tel: |
82- 2 -783 - 8063 |
| Website: |
www.chemicalland21.com |
|