| Identification | Back Directory | [Name]
Ivabradine | [CAS]
155974-00-8 | [Synonyms]
Ivabradine
Ivabridine –
Ivabradine API
Ivabradine sulfate
Ivabradine USP/EP/BP
Ivabradine HCl Premix
Ivabradine(Hydrochloride form)
3-[3-[[(8S)-3,4-dimethoxy-8-bicyclo[4.2.0]octa-1,3,5-trienyl]methyl-methylamino]propyl]-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-one
3-[3-[[(8S)-3,4-dimethoxy-8-bicyclo[4.2.0]octa-1,3,5-trienyl]methyl-methyl-amino]propyl]-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-one
7-(3-((((R)-4,5-diMethoxy-1,2-dihydrocyclobutabenzen-1-yl)Methyl)(Methyl)aMino)propyl)-2,3-diMethoxy-8,9-dihydro-5H-benzo[7]annulen-6(7H)-one
2H-3-Benzazepin-2-one, 3-[3-[[[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl]methylamino]propyl]-1,3,4,5-tetrahydro-7,8-dimethoxy-
IvabradineQ: What is
Ivabradine Q: What is the CAS Number of
Ivabradine Q: What is the storage condition of
Ivabradine Q: What are the applications of
Ivabradine | [Molecular Formula]
C27H36N2O5 | [MDL Number]
MFCD04975447 | [MOL File]
155974-00-8.mol | [Molecular Weight]
468.59 |
| Hazard Information | Back Directory | [Description]
Ivabradine is a first selective and specific If inhibitor that
was approved by EMEA in November for symptomatic
treatment of chronic stable angina pectoris in patients with
normal sinus rhythm. This is the first agent to lower heart
rate by inhibiting the cardiac pacemaker If current. The compound
was discovered and developed by Servier and is currently
being marketed in Ireland. | [Uses]
Ivabradine is a potent and orally active HCN (hyperpolarization-activated cyclic nucleotide-gated) channel blocker that inhibits the cardiac pacemaker current (If). Ivabradine reduces dose-dependently heart rate without modification of blood pressure. Ivabradine shows anticonvulsant, anti-ischaemic and anti-anginal activity[1][2][3][4]. | [Definition]
ChEBI: A member of the class of benzazepines that is 7,8-dimethoxy-1,3,4,5-tetrahydro-3-benzazepin-2-one in which the amide hydrogen is replaced by a [{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}(methyl)amino]propyl} group. Use
(as its hydrochloride salt) to treat patients with angina who have intolerance to beta blockers and/or heart failure. | [Synthesis]
The convergent synthesis
of ivabradine was accomplished by coupling the key
benzocylclobutanyl amine 73 with oxadioxalane 76 in an in
situ deprotection and amination as shown in Scheme 13.
For the synthesis of the key amine 73, cyano group of compound
69 is reduced with borane-THF to give amine 70 in
90% yield, which was reacted with ethyl chloroformate to
give carbamate 71 in 80% yield. Complete reduction of the
carbamate was accomplished by refluxing with LAH in THF
to give racemic methyl amine 72 in 92% yield, which was
then resolved by crystallizing with N-acetyl ¨CL-glutamic acid to give chiral salt 73. Prior to the next step, the amine is
converted to the hydrochloride salt.
The coupling partner 76 to make ivabradine was prepared
from the azepinone 74 by first reacting with bromoethyldioxalane
to give 75. The olefin in 75 was reduced by hydrogenating
with palladium/carbon catalyst at 55??C to give 76.
To the same pot, the amine 73 was added and hydrogenated
to give reductive amination product ivabradine hydrochloride
(X) in very good yields.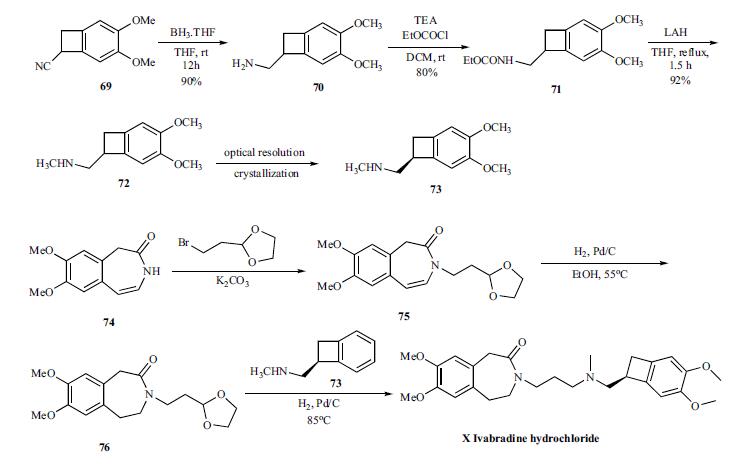
| [in vivo]
Ivabradine (1, 10, 20 mg/kg; i.p.) shows anticonvulsant and neuroprotective action in mice[3].
Ivabradine (5, 10, 20 mg/kg;p.o.; daily for 1 weeks) lowers heart rate in mice with enhanced sympathoadrenergic activities[4]. | Animal Model: | 25-30 g, 6 weeks male Swiss mice[3] | | Dosage: | 1, 10, 20 mg/kg | | Administration: | I.p.; for 3 days | | Result: | Attenuated PTZ- and PICRO-induced seizures while presented an antioxidant effect in all
brain areas studied, and reduced cleaved caspase-3 expression in the CA1 and DG region of PICRO- and PTZ-treated mice, respectively. |
| Animal Model: | 3-4 months transgenic (TG) mice with cardiac-restricted overexpression of b2AR[4] | | Dosage: | 5, 10, 20 mg/kg | | Administration: | P.o; daily for 1 weeks | | Result: | Reduced the maximal HR increase in response to the b-agonist isoproterenol, without modifying the response of contractile parameters at 10 mg/kg. |
| [References]
[1] Tardif JC, et al. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005 Dec;26(23):2529-36. DOI:10.1093/eurheartj/ehi586
[2] Mulder P, et al. Heart rate slowing for myocardial dysfunction/heart failure. Adv Cardiol. 2006;43:97-105. DOI:10.1159/000095431
[3] Cavalcante TMB, et al. Ivabradine possesses anticonvulsant and neuroprotective action in mice. Biomed Pharmacother. 2019 Jan;109:2499-2512. DOI:10.1016/j.biopha.2018.11.096
[4] Du XJ, et al. I(f) channel inhibitor ivabradine lowers heart rate in mice with enhanced sympathoadrenergic activities. Br J Pharmacol. 2004 May;142(1):107-12. DOI:10.1038/sj.bjp.0705696 |
|
|