| Identification | Back Directory | [Name]
HEXAFLUOROCYCLOTRIPHOSPHAZENE | [CAS]
15599-91-4 | [Synonyms]
AURORA KA-1097
HEXAFLUOROCYCLOTRIPH
HEXAFLUOROCYCLOTRIPHO
Phosphonitrilefluoridetrimer
HEXAFLUOROCYCLOTRIPHOSPHAZENE
PHOSPHONITRILIC FLUORIDE TRIMER
Hexafluorocyclotriphosphazene>
tris(phosphorusnitridedifluoride)
1,3,5,2,4,6-Triazatriphosphorine, hexafluoro-
1,3,5-TRIAZA-2,4,6-TRIPHOSPHORIN-2,2,4,4,6,6-HEXAFLUORIDE
1,2,3,4,4,6-hexafluoro-1,2,3,6-tetrahydro-1,3,5,2,4,6-triazatriphosphinine
1,3,5,2,4,6-Triazatriphosphorine, 2,2,4,4,6,6-hexafluoro-2,2,4,4,6,6,-hexahydro-
1,3,5-Triaza-2,4,6-triphosphorin-2,2,4,4,6,6-hexafluoride, Hexafluorocyclotriphosphazene | [Molecular Formula]
F6N3P3 | [MDL Number]
MFCD00192400 | [MOL File]
15599-91-4.mol | [Molecular Weight]
248.93 |
| Chemical Properties | Back Directory | [Melting point ]
25-30 °C(lit.)
| [Boiling point ]
50.9 °C(lit.)
| [density ]
2.237 g/cm3 (20 ºC ) | [refractive index ]
1.3183 (589.3 nm 32℃) | [storage temp. ]
2-8°C
| [solubility ]
soluble in Toluene | [form ]
powder to lump | [pka]
-27.34±0.27(Predicted) | [color ]
White to Almost white |
| Hazard Information | Back Directory | [Uses]
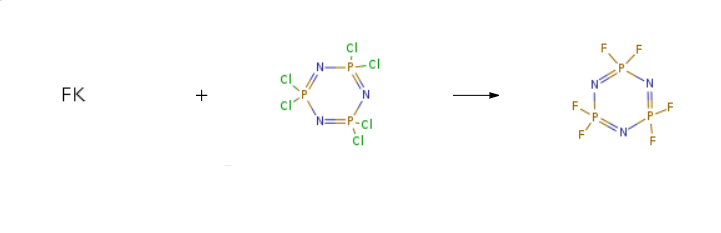
Hexafluorocyclotriphosphazene is an intermediate for organic materials, which can be used as a raw material for the synthesis of other components or electronic materials, and can be used in the preparation of spiro ethers and flame retardant additives for lithium ion batteries. | [Synthesis]
Hexafluorocyclotriphosphazene is prepared by the reaction of potassium fluoride and 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine. The steps are as follows:
Put 104.3g of hexachlorocyclotriphosphazene, 113.3g of potassium fluoride, 0.011g of ionic liquid catalyst [Nbmm]OH, and 217.6g of anhydrous acetonitrile in an electric stirrer, thermometer, In the flask with reflux condenser, the reaction was carried out at 30°C, and the reaction was closed after 2h, filtered, The filtrate was rectified to obtain hexafluorocyclotriphosphazene with a yield of 98.7%.
 |
|
|