| Identification | Back Directory | [Name]
5-Bromo-7-methyl-1H-indole | [CAS]
15936-81-9 | [Synonyms]
EOS-61850
5-BroMo-7-Methylindole
7-methyl-5-bromo-1H-indole
5-Bromo-7-methyl-1H-indole
1H-Indole, 5-bromo-7-methyl- | [Molecular Formula]
C9H8BrN | [MDL Number]
MFCD12962648 | [MOL File]
15936-81-9.mol | [Molecular Weight]
210.07 |
| Chemical Properties | Back Directory | [Boiling point ]
165 °C(Press: 0.1 Torr) | [density ]
1.503 g/mL at 25 °C | [refractive index ]
n20/D1.648 | [Fp ]
>110℃ | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
Solid or semi-solid or lump or liquid | [pka]
16.34±0.30(Predicted) |
| Hazard Information | Back Directory | [Synthesis]
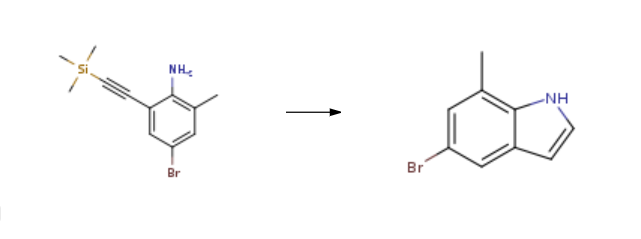
5-Bromo-7-methyl-1H-indole is synthesised from 4-bromo-6-(trimethylsilylacetylenyl)-2-methylaniline by reacting with potassium tert-butyrate and sodium hydride in the presence of inert gas. The steps are as follows:
Add sodium hydride (2.64g, 0.11mol) and DMF (70mL) to a 250ml three-necked flask at room temperature and under nitrogen protection.Stir and dissolve; a solution of the compound of formula IV (14g, 0.05mol) dissolved in DMF (30mL) is slowly added dropwise to the potassium tert-butoxide solution. After the addition, the reaction system was heated to 60 degrees and kept for 2 hours. After the reaction was completed, the reaction solution was poured into ice water, extracted with methyl tert-butyl ether, and washed with sodium bicarbonate aqueous solution and brine in turn.It was then dried over anhydrous sodium sulfate, filtered, and concentrated. The crude product is purified by silica gel column chromatography to obtain the compound of 5-bromo-7-methylindole. Yield: 8.36 g, yield: 80%.
|
|
| Company Name: |
Stereokem Pvt Ltd
|
| Tel: |
+91-4029706929 +91-9394224843 |
| Website: |
www.stereokem.com |
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
NovoChemy Ltd.
|
| Tel: |
021-31261262/ 49 (0)17662837245 |
| Website: |
www.novochemy.com |
|