| Identification | Back Directory | [Name]
Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride | [CAS]
16090-14-5 | [Synonyms]
PSVE
FS 141
Perfluoro(sulfonylvinyl)ether
2,2-tetrafluoro-etrafluoroethoxy]-1
PERFLUORO-2-(2-FLUOROSULPHONYLETHOXY)PROPYLVINYLETHER
PERFLUORO-2-(2-FLUOROSULFONYLETHOXY)PROPYL VINYL ETHER
PERFLUORO(4-METHYL-3,6-DIOXAOCT-7-ENE)SULFONYL FLUORIDE
Perfluoro-3,6-dioxa-4-methyloct-7-enesulphonyl fluoride
perfluoro(4-methyl-3,6-dioxa-7-octene)sulfonyl fluoride
PERFLUORO(4-METHYL-3,6-DIOXAOCT-7-ENE)SULPHONYL FLUORIDE
Perfluoro-2-(2-fluorosulfonylethoxy) Propyl Vinyl Ether (PSEPVE
4-(Trifluoromethyl)decafluoro-3,6-dioxa-7-octene-1-sulfonic acid fluoride
ethanesulfonylfluoride,2-[1-[difluoro[(trifluoroethenyl)oxy]methyl]-1,2,2,2-t
1,1,2,2,4,5,5,7,8,8-Decafluoro-4-(trifluoromethyl)-3,6-dioxa-7-octene-1-sulfonyl fluoride
1,1,2-Trifluoro-2-[1,1,2,3,3,3-hexafluoro-2-[1,1,2,2-tetrafluoro-2-(fluorosulfonyl)ethoxy]propoxy]ethene
2-[1-(Trifluoromethyl)-2-[(trifluorovinyl)oxy]trifluoroethoxy]-1,1,2,2-tetrafluoroethane-1-sulfonyl fluoride
2-({1,1,1,2,3,3-Hexafluoro-3-[(trifluoroethenyl)oxy]prop-2-yl}oxy)-1,1,2,2-tetrafluoroethanesulphonyl fluoride
1,1,2,2-tetrafluoro-2-[1,1,1,2,3,3-hexafluoro-3-(1,2,2-trifluoroethenoxy)propan-2-yl]oxyethanesulfonyl fluoride
1,1,2,2-Tetrafluoro-2-[1,2,2-trifluoro-1-(trifluoromethyl)-2-[(trifluorovinyl)oxy]ethoxy]ethanesulfonyl fluoride
1,1,2,2-tetrafluoro-2-[1,2,2-trifluoro-1-(trifluoromethyl)-2-[(trifluorovinyl)oxy]ethoxy]ethanesulphonyl fluoride
Ethanesulfonylfluoride, 1,1,2,2-tetrafluoro-2-[1,2,2-trifluoro-1-(trifluoroMethyl)-2-[(trifluorovinyl)oxy]ethoxy]-
2-[2-(Trifluorovinyloxy)-1-(trifluoromethyl)-1,2,2-trifluoroethoxy]-1,1,2,2-tetrafluoroethanesulfonic acid fluoride
2-{1-{Difluoro-[(trifluoroethenyl)-oxy]-methyl}-1,2,2,2-tetrafluoroethoxy}-1,1,2,2-tetrafluoroethanesulfonyl fluoride
1,1,2,2-Tetrafluoro-2-((1,1,1,2,3,3-hexafluoro-3-((1,2,2-trifluorovinyl)oxy)propan-2-yl)oxy)ethane-1-sulfonyl fluoride
Ethanesulfonyl fluoride, 2-[1-[difluoro[(1,2,2-trifluoroethenyl)oxy]methyl]-1,2,2,2-tetrafluoroethoxy]-1,1,2,2-tetrafluoro-
1,1,2,2-Tetrafluoro-2-[1,2,2-trifluoro-1-(trifluoromethyl)-2-(1,2,2-trifluoroethenyloxy)ethoxy]-1-ethanesulfonic acid fluoride | [EINECS(EC#)]
240-249-4 | [Molecular Formula]
C7F14O4S | [MDL Number]
MFCD00798138 | [MOL File]
16090-14-5.mol | [Molecular Weight]
446.12 |
| Chemical Properties | Back Directory | [Boiling point ]
135°C | [density ]
1,7 g/cm3 | [storage temp. ]
Refrigerator, under inert atmosphere | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Oil | [color ]
Colourless | [EPA Substance Registry System]
Ethanesulfonyl fluoride, 2-[1-[difluoro[(trifluoroethenyl)oxy]methyl]-1,2,2,2-tetrafluoroethoxy]-1,1,2,2-tetrafluoro- (16090-14-5) |
| Hazard Information | Back Directory | [Uses]
Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes. | [Synthesis]
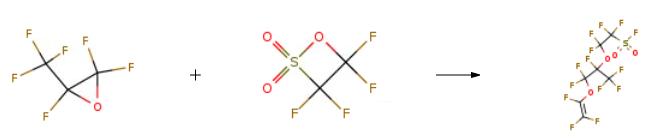
(1) Intermediate synthesis In a 1L reactor, add 100ml of dimethylacetamide, 8g of potassium fluoride, and 200g (1.11mol) of tetrafluoroethane-|?-sultone,Subsequently, 500g (3mol) of hexafluoropropylene oxide was introduced at a flow rate of 0.3kg/h for addition reaction, and the reaction temperature was controlled at 20??C.The reaction pressure is controlled at 0.4MPa. After the introduction of hexafluoropropylene oxide is completed, the reaction is continued for 1 hour. After the reaction is completed,The reaction liquid was rectified to obtain 528g (1.03mol) of intermediate, with a yield of 92% and a purity of 99.1%;(2) PSVE synthesis In a moving bed reactor (volume 2L, material 316L, produced by Tianjin Qixi Technology Co., Ltd.),A mixture of 500g (0.97mol) of the intermediate obtained in step (1) and 600g of potassium carbonate (4.34mol) was passed through the salt formation zone and decarboxylation zone of the moving bed reactor in turn (the salt formation temperature was set to 150??C,The decarboxylation temperature is set to 360??C) to carry out the salt formation reaction and the decarboxylation reaction, and the contact time of the salt formation reaction is 10 min by controlling the material feed rate.The contact time of the decarboxylation reaction is 25min, and the decarboxylation reaction product is collected by condensation to obtain a crude product.The crude product is rectified to obtain the product perfluoro-3,6-dioxa-4-methyl-7-octenesulfonyl fluoride with a purity of 98.5% and a yield of 85%. |
|
|