| Identification | Back Directory | [Name]
Methyl 2-(hydroxymethyl)nicotinate | [CAS]
1697289-97-6 | [Synonyms]
Methyl 2-(hydroxymethyl)nicotinate
2-Hydroxymethyl-nicotinic acid methyl ester
3-Pyridinecarboxylic acid, 2-(hydroxymethyl)-, methyl ester | [Molecular Formula]
C8H9NO3 | [MDL Number]
MFCD28578559 | [MOL File]
1697289-97-6.mol | [Molecular Weight]
167.16 |
| Hazard Information | Back Directory | [Description]
Methyl 2-(hydroxymethyl)nicotinate(1697289-97-6) is a derivative of methyl nicotinate, which is used in the preparation of a novel glycosidase inhibitor, 1-azasugars. Methyl 2-(hydroxymethyl)nicotinate and its derivatives are used in the preparation of haemoglobin modulators[1].
| [Uses]
Methyl 2-(hydroxymethyl)nicotinate(1697289-97-6) can be used as pharmaceutical intermediate for laboratory research.
| [Synthesis]
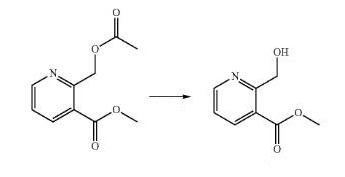
To
a
solution
of
methyl
2-(acetoxymethyl)nicotinate
(7.90
g,
37.76
mmol,
1.0
equiv),
in
MeOH
(80
mL)
was
added
acetyl
chloride
(3.60
g,
45.86
mmol,
1.2
equiv).
The
reaction
solution
was
stirred
overnight
at
room
temperature;
then,
the
solvent
was
removed
under
reduced
pressure,
and
the
resulting
residue
was
dissolved
in
water
(20
mL).
The
pH
was
adjusted
to
8
with
NaHCO
3
solid
and
extracted
with
ethyl
acetate
(30
mL*3).
The
combined
organic
phase
was
dried
over
anhydrous
sodium
sulfate
and
filtered,
and
the
filtrate
was
concentrated
in
vacuum.
The
residue
was
puri
-
fied
by
silica
gel
column
with
ethyl
acetate/petroleum
ether
(1/1),
giving
Methyl 2-(hydroxymethyl)nicotinate.
LCMS
(ES)
[M+1]
+
m/z:
168.
 | [References]
[1] GUOHUA ZHAO; Bruce G; Urmila C Deo. Selective Fowler Reductions: Asymmetric Total Syntheses of Isofagomine and Other 1-Azasugars from Methyl Nicotinate[J]. Organic Letters, 2000. DOI:10.1021/ol006810x. |
|
| Company Name: |
Cochemical Ltd.
|
| Tel: |
029-86115547 17791676824 |
| Website: |
www.cochemical.com |
| Company Name: |
Synthonix Inc
|
| Tel: |
001-9198759277 |
| Website: |
www.synthonix.com |
|