| Identification | Back Directory | [Name]
TRANS-2-PHENYLCYCLOPROPYLAMINE HYDROCHLORIDE | [CAS]
1986-47-6 | [Synonyms]
SKF-385 HCl
TRANYLCYPROMINE
TIMTEC-BB SBB003859
SKF-385 hydrochloride
TRANYLCYPROMINE HYDROCHLORIDE
Tranylcyprominie hydrochloride
Terephathalic acid monoethyl ester
TRANS-2-PHENYLCYCLOPROPYLAMINE HCL
trans-TranylcyproMine Hydrochloride
(DL)-Trans-2-phenylcyclopropylamine
Tranylcypromine (2-PCPA) hydrochloride
rac trans-2-PhenylcyclopropylaMine HCl
TRANS-2-PHENYLCYCLOPROPYLAMINE HYDROCHLORIDE
trans-2-PhenylcyclopropanaMine hydrochloride
(1R,2S)-2-phenylcyclopropanaMine hydrochloride
trans-2-Phenylcyclopropylamine hydrochloride,97%
rac trans 2-Phenylcyclopropylamine Hydrochloride
trans-2-PhenylcyclopropylaMine hydrochloride 97%
trans 2-Amino-1-phenyl-cyclopropane Hydrochloride
(1R,2S)-rel-2-PhenylcyclopropanaMine hydrochloride
trans-2-phenylcyclopropylaMine hydrochloride >97% ( GC), %
trans-2-Phenylcyclopropylamine hydrochloride,Tranylcypromine | [Molecular Formula]
C9H12ClN | [MDL Number]
MFCD00063602 | [MOL File]
1986-47-6.mol | [Molecular Weight]
169.65 |
| Chemical Properties | Back Directory | [Appearance]
white to light beige powder or chunks | [Melting point ]
162-169 °C(lit.)
| [storage temp. ]
2-8°C
| [solubility ]
Methanol (Slightly), Water (Slightly) | [form ]
Powder or Chunks | [color ]
White to light beige | [biological source]
synthetic (organic) | [optical activity]
[α]/D 1 to +1.0°, c = 1 in H2O | [Stability:]
Hygroscopic |
| Hazard Information | Back Directory | [Chemical Properties]
white to light beige powder or chunks | [Uses]
Antidepressant;MAO inhibitor | [Uses]
Non-selective MAO-A/B inhibitor | [Biological Activity]
Irreversible inhibitor of lysine-specific demethylase 1 (LSD1/BHC110) and monoamine oxidase (MAO) (K i values are 242, 102 and 16 μ M for LSD1, MAO-A and MAO-B respectively). Inhibits histone demethylation. | [Definition]
ChEBI: (1R,2S)-tranylcypromine hydrochloride is a hydrochloride obtained by combining (1R,2S)-tranylcypromine with one equivalent of hydrochloric acid. It contains a (1R,2S)-tranylcypromine(1+). It is an enantiomer of a (1S,2R)-tranylcypromine hydrochloride. | [General Description]
A cell-permeable phenylcyclopropylamine that inhibits the monoamine oxidase and histone demethylase activities, respectively, of MAO A/B (Ki = 101.9 and 16.0 M, respectively) and LSD1/2 (Ki = 242.7 and 180.0 M, respectively), four members of a flavin-dependent amine oxidase family enzymes, by a covalent adduct formation with the enzyme-bound FAD. In addition to preventing LSD1-CoREST (Corepressor of RE1-Silencing Transcription factor) complex-mediated H3K4 demethylation (IC50<2 M), TCP also inhibits LSD1-HCF-1 (Host Cell Factor-1) complex-mediated H3K9 demethylase activity, which is demonstrated to be an essential mechanism for the replication and latent infection of the α-herpesviruses HSV and VZV. The combined treatment of 2 M TCP and 10 M CHIR99061 is reported to enable the reprogramming of Oct4/Klf4-transduced primary HNEKs (Human Neonatal Epidermal Keratinocytes) into iPS (induced Pluripotent Stem) cells, albeit at a 100-time lower efficiency as seen in cultures transduced with 4-TFs (Oct44, Klf4, Sox2, and c-Myc). | [Clinical Use]
MAOI antidepressant | [Synthesis]
Tranylcypromine, (?à)-trans-2-phenylcyclopropylamine (7.2.10),
differs from the drugs described above in that it is not a derivative of hydrazine. It is
synthesized from the ethyl ester of 2-phenylcyclopropan carboxylic acid (7.2.7), which is synthesized by the reaction of styrene with ethyl diazoacetate. 2-phenylcyclopropan�carboxylic acid ethyl ester (7.2.7) is hydrolyzed by alkali to 2-phenylcyclopropancar�boxylic acid (7.2.8) and the trans-isomer is separated for further reactions. The reaction
of the trans-isomer with thionyl chloride gives trans-2-phenylcyclopropancarboxylic
acid chloride (7.2.9), which upon reaction with sodium azide gives the respective acid
azide, which undergoes Curtius rearrangement to the transcyclopropylamine (7.2.10)
[48,49].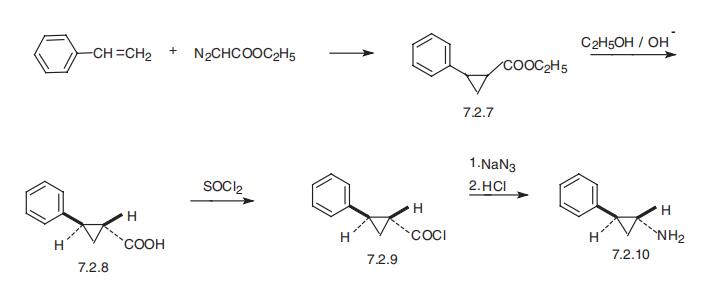
| [Drug interactions]
Potentially hazardous interactions with other drugs
Alcohol: some alcoholic and dealcoholised drinks
contain tyramine which can cause hypertensive crisis.
Alpha-blockers: enhanced hypotensive effect; avoid
with indoramin.
Analgesics: CNS excitation or depression with
pethidine, other opioids and nefopam - avoid;
increased risk of serotonergic effects and convulsions
with tramadol - avoid.
Antibacterials: increased risk of hypertension and
CNS excitation with linezolid and tedizolid - avoid
for at least 2 weeks after stopping MAOIs.
Antidepressants: enhancement of CNS effects and
toxicity. Care with all antidepressants including drug
free periods when changing therapies.
Antidiabetics: possibly enhanced hypoglycaemic
effect.
Antiepileptics: antagonism of anticonvulsant effect;
avoid carbamazepine with or within 2 weeks of
MAOIs.
Antihypertensives: enhanced hypotensive effect.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Antipsychotics: effects enhanced by clozapine.
Anxiolytics: avoid buspirone with or within 2 weeks
of MAOIs.
Atomoxetine: avoid concomitant use and for 2 weeks
after use; increased risk of convulsions.
Bupropion: avoid with or for 2 weeks after MAOIs.
Dapoxetine: risk of hypertensive crisis - avoid.
Diuretics: enhanced hypotensive effect; avoid with
indoramin.
Dopaminergics: avoid with entacapone, safinamide
and tolcapone; hypertensive crisis with levodopa and
rasagiline - avoid for at least 2 weeks after stopping
MAOI; hypotension with selegiline.
5HT1
agonist: risk of CNS toxicity with
sumatriptan, rizatriptan and zolmitriptan - avoid
sumatriptan and rizatriptan for 2 weeks after MAOI.
Metaraminol: risk of hypertensive crisis - avoid for
at least 2 weeks after stopping MAOIs.
Methyldopa: avoid concomitant use.
Opicapone: avoid concomitant use.
Sympathomimetics: hypertensive crisis with
sympathomimetics - avoid.
Tetrabenazine: risk of CNS excitation and
hypertension avoid. | [Metabolism]
Tranylcypromine undergoes considerable hepatic metabolism, including breakdown of the side chain and probably conjugation. Excretion is renal mainly as metabolites. | [storage]
room temperature (desiccate) |
|
|