| Identification | Back Directory | [Name]
2-Ethyl-2-adamantyl methacrylate | [CAS]
209982-56-9 | [Synonyms]
2-ETHYL-2-ADAMANTYL METHACRYLA
2-Ethyl-2-adamantyl methacrylate
2-Ethyladamant-2-yl methacrylate
2-Ethyl-2-Adamanthyl Methacrylate
2-Ethyl-2-methacryloyloxyadamantane
2-ethacryloyloxy-2-methyladamantane
EAMA 2-Ethyl-2-adamantyl methacrylate
Methacrylic Acid 2-Ethyl-2-adamantyl Ester
2-Ethyl-2-adamantyl methacrylate 209982-56-9
2-Ethyl-2-methacryloyloxyadamantane 209982-56-9
2-Ethyl-2-adamantyl methacrylate EAMA ArF monomers
2-Ethyl-2-methacryloyloxyadamantane(stabilizedwithMEHQ)>
2-Ethyl-2-MethacryloyloxyadaMantane (stabilized with MEHQ)
2-Ethyltricyclo[3.3.1.1~3,7~]dec-2-yl 2-methylprop-2-enoate, EADM
2-Propenoic acid, 2-methyl-, 2-ethyltricyclo[3.3.1.13,7]dec-2-yl ester
2-Ethyl-2-adamantyl Methacrylate
Methacrylic Acid 2-Ethyl-2-adamantyl Ester | [Molecular Formula]
C16H24O2 | [MDL Number]
MFCD06411144 | [MOL File]
209982-56-9.mol | [Molecular Weight]
248.36 |
| Hazard Information | Back Directory | [Uses]
2-Ethyl-2-adamantyl methacrylate(209982-56-9) is a monomer used in the synthesis of organic compounds.
| [Application]
2-Ethyl-2-adamantyl methacrylate (EADMA,209982-56-9) is mainly used as photoresist ArF monomer for the preparation of photoresist materials. PAG-bound polymer resists (HS-EA-PAG and GB-EA-PAG) prepared from EADMA and hydroxystyrene (HOST) or γ-butyrolactone methacrylate (GBLMA) are effective resists for 193 nm or EUV lithography.
| [Preparation]
The preparation of 2-Ethyl-2-adamantyl methacrylate(209982-56-9) is as follows:Add 2175ml of n-hexane to the 3L four-necked flask equipped with stirring paddle, condenser tube and thermometer, turn on stirring, add 97g of 2-ethyladamantanol to the system, and slowly add 0.0072g of polymerization inhibitor tetrachlorobenzoquinone to the reaction system , add 72.5g of basic ion exchange resin, adjust pH=7-8, cool down to -10°C, slowly add 72.5g of vinyl methacrylate dropwise to the reaction system, keep the reaction for 6h, monitor the reaction progress, take a sample to detect the reaction is complete After adding 800 ml of water to the reaction system, stirring and extracting the phases, the organic phase was dried, concentrated under reduced pressure and evaporated to dryness to obtain 125 g of 2-ethyl-2-adamantyl methacrylate product with a yield of 93.45%.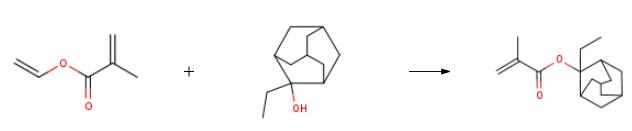
| [General Description]
Because adamantane has optical properties, heat resistance, and other properties, its derivatives are often used in optical disc substrates, optical fibers, or lens lamps. It can be used as a semiconductor photoresist, sealant for optical semiconductors, optical-electronic components, and their adhesives. Among them, electronic components made from alkyl adamantyl acrylates as raw materials have high resistance to dry etching in the semiconductor manufacturing process and have broad development prospects as semiconductor materials.
|
|
|