| Identification | Back Directory | [Name]
Mirabegron | [CAS]
223673-61-8 | [Synonyms]
YM 178
CS-732
Mirabelon
Mirabello
Mirabregon
Mira veron
MIRABEQRON
Mirabegron
Mirabegron, >=99%
Mirabegron(YM 178)
Mirabegron USP/EP/BP
Merariveron impurity B
hot selling Mirabegron
Mirabegron Intermediates
(R)-2-(2-aminothiazol-4-yl)-4'-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl}acetanilide
N-(4-(2-(2-hydroxy-2-phenylethylamino)ethyl)phenyl)-2-(2-aminothiazol-4-yl)acetamide
2-AMino-N-[4-[2-[[(2R)-2-hydroxy-2-phenyl-ethyl]aMino]-ethyl]phenyl]-4-thiazoleacetaMide
(R)-2-(2-aminothiazol-4-yl)-N-(4-(2-(2-hydroxy-2-phenylethylamino)ethyl)phenyl)acetamide
4-Thiazoleacetamide, 2-amino-N-(4-(2-(((2R)-2-hydroxy-2-phenylethyl)amino)ethyl)phenyl)-
2-(2-aMino-1,3-thiazol-4-yl)-N-(4-{2-[(2-hydroxy-2-phenylethyl)aMino]ethyl}phenyl)acetaMide
(2R)-2-(2-AMinothiazol-4-yl)-4'-[2-[(2-hydroxy-2-phenylethyl)aMino]ethyl]acetic Acid Anilide
2-(2-Amino-1,3-thiazol-4-yl)-N-[4-(2-{[(2R)-2-hydroxy2-phenylethyl]amino}ethyl)phenyl]acetamide | [EINECS(EC#)]
800-126-3 | [Molecular Formula]
C21H24N4O2S | [MDL Number]
MFCD11100356 | [MOL File]
223673-61-8.mol | [Molecular Weight]
396.506 |
| Chemical Properties | Back Directory | [Melting point ]
138-140°C | [Boiling point ]
690.0±55.0 °C(Predicted) | [density ]
1.313 | [storage temp. ]
-20°C Freezer | [solubility ]
Chloroform (Slightly, Heated), Methanol (Slightly) | [form ]
Solid | [pka]
13.51±0.70(Predicted) | [color ]
White to Pale Yellow | [InChIKey]
PBAPPPCECJKMCM-IBGZPJMESA-N | [SMILES]
S1C=C(CC(NC2=CC=C(CCNC[C@H](O)C3=CC=CC=C3)C=C2)=O)N=C1N | [CAS DataBase Reference]
223673-61-8 |
| Hazard Information | Back Directory | [Description]
Betanis (Mirabegron) was approved in July 2011 by the Japanese
Ministry of Health, Labour, and Welfare for the treatment of urgency,
urinary frequency, and urinary urge urinary incontinence associated with
overactive bladder (OAB).
Mirabegron is synthesized by coupling 4-nitrophenethyl amine to (R)-2-hydroxy-2-phenylacetic acid. The resulting amide is reduced to an amine. The nitro group is then
reduced and the resulting aniline is coupled to 2-(2-aminothiazol-4-yl)
acetic acid to give mirabegron. Mirabegron has an EC50 of 22 nM (intrinsic activity=0.8) for β3-AR with no detectable activity for β1- andβ2-AR (EC50>10,000 nM). In an anesthetized rat rhythmic bladder
contraction model in which bladder contractions are induced by saline,
mirabegron at 3 mg/kg iv decreased the frequency of rhythmic bladder
contraction without suppressing contraction amplitude. These data suggest
that the activation of β3-AR increases bladder capacity without
influencing the frequency of bladder contraction. | [Chemical Properties]
White to Off-White Solid | [Originator]
Astellas Pharma Inc. (Japan) | [Uses]
A potent bladder relaxant compound | [Uses]
Mirabegron is a selective β3-adrenoceptor agonist with EC50 of 22.4 nM. | [Uses]
Potent bladder relaxant and reagent for diabetes remedy.;Labeled Mirabegron, intended for use as an internal standard for the quantification of Mirabegron by GC- or LC-mass spectrometry. | [Definition]
ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of 2-amino-1,3-thiazol-4-ylacetic acid with the anilino group of (1R)-2-{[2-(4-aminophenyl)ethyl]amino}-1-phenylethanol. Used for the treatment of overactive
ladder syndrome. | [Brand name]
Betanis | [Clinical Use]
Mirabegron is an orally active β3-adrenoceptor agonist currently
in development by Astellas Pharma for the treatment of overactive
bladder (OAB). The drug is a nanomolar EC50 antagonist against human
β3-AR biochemical assays with good selectivity over b1- and
β2-ARs. Mirabegron demonstrates a novel mechanism by targeting
the β3-AR for bladder relaxation to help manage OAB symptoms
such as increased urinary urgency and frequency and urgency incontinence.
However, mirabegron is a cytochrome P450 2D6 inhibitor,
and it raises a concern for drug–drug interaction with concomitant
administration of other cytochrome P450 2D6 substrates. | [Synthesis]
The synthesis of mirabegron began with a condensation
reaction between (R)-styrene oxide (182) and 4-nitrophenylethylamine
(183) in refluxing isopropanol to yield corresponding
aminoalcohol 184 in 22% yield. Aminoalcohol 184
was protected as its N-Boc derivative with t-butyl dicarbonate
in THF in 96% yield, and this was followed by nitro group
hydrogenative reduction with 10% Pd/C to give free amine 185
in 96% yield. Aniline 185 was coupled with 2-amino-4-thiazolyl
acetic acid 186 in the presence of EDCI and HOBt to give amide
187 in 85% yield. Removal of the Boc group was affected with
4 N HCl solution in a 2:1 volume ratio in ethyl acetate to obtain
mirabegron HCl in 52% yield. The HCl salt was neutralized with
1 N NaOH to deliver mirabegron (XVII).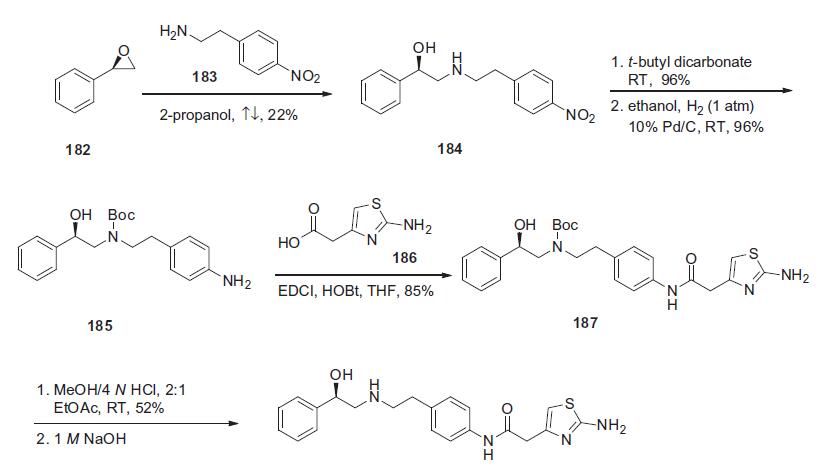
| [Drug interactions]
Potentially hazardous
interactions with other drugs
None known | [Metabolism]
Metabolised via multiple pathways involving dealkylation, oxidation, (direct) glucuronidation, and amide hydrolysis. Renal elimination of mirabegron is primarily through active tubular secretion along with glomerular filtration. | [storage]
Store at -20°C |
|
|