| Identification | Back Directory | [Name]
1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE | [CAS]
2547-66-2 | [Synonyms]
LABOTEST-BB LT00138201
1,3,5-Tribenzyl-1,3,5-triazinane
1,3,5-TRIBENZYLHEXAHYDRO-S-TRIAZINE
1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE
Hexahydro-1,3,5-tribenzyl-1,3,5-triazine
1,3,5-Tribenzylhexahydro-1,3,5-triazine 98%
1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE 98+%
Hexahydro-1,3,5-tris(phenylmethyl)-1,3,5-triazine
1,3,5-Triazine, hexahydro-1,3,5-tris(phenylmethyl)-
1,3,5-Tribenzylhexahydro-1,3,5-triazine, 98+%
white powder | [EINECS(EC#)]
219-831-7 | [Molecular Formula]
C24H27N3 | [MDL Number]
MFCD00014599 | [MOL File]
2547-66-2.mol | [Molecular Weight]
357.49 |
| Chemical Properties | Back Directory | [Melting point ]
49-51 °C(lit.)
| [Boiling point ]
100 °C0.005 mm Hg(lit.)
| [density ]
113 | [refractive index ]
1.6270 (estimate) | [Fp ]
>230 °F
| [storage temp. ]
Sealed in dry,Room Temperature | [form ]
solid | [pka]
5.46±0.20(Predicted) | [InChIKey]
VWVZIRPJPFJGFE-UHFFFAOYSA-N |
| Questions And Answer | Back Directory | [Uses]
1,3,5-Tribenzyl-1,3,5-triazinane is used as a reagent in the synthesis of phosphinic acid based N-Acetylated alpha-linked acidic dipeptidase (NAALADase) inhibitors, which may potentially be used for the treatment of both neurodegenerative disorders and peripheral neuropathies.
|
| Hazard Information | Back Directory | [Application]
1,3,5-Tribenzylhexahydro-1,3,5-triazine was used:in the synthesis of alkyl β-aminocarboxylates via trifluoromethanesulfonic acid catalyzed reaction with ketene silyl acetals;as ligand to study the assembling process of a heterogeneous Cr-based single-site ethylene trimerization catalyst by X-ray absorption spectroscopy;as ligand during conversion of Phillips Cr/SiO2 polymerisation catalyst to ethylene trimerisation catalyst after assembling new active sites on the silica surface. | [Synthesis]
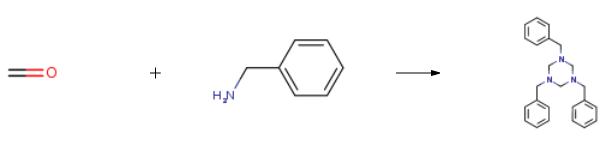
To a round-bottomed flask (125 mL) equipped with a reflux condenser was added theappropriate amine (0.05 mmol), toluene (40 mL) and formaldehyde (37%, 4.1 mL).The solution was brought to reflux using an external oil bath and kept stirring for 30min. Then, the toluene was evaporated under reduced pressure, and the residue wasdissolved in ethyl acetate and washed with a saturated aqueous solution of sodiumchloride. After evaporation of the solvent under reduced pressure in a rotaryevaporator, the residue was purified by silica gel column chromatography usinghexane/ethyl acetate 9:1 as the eluent. |
| Spectrum Detail | Back Directory | [Spectrum Detail]
1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE(2547-66-2)1HNMR
1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE(2547-66-2)IR
1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE(2547-66-2)Raman
|
|
|