| Identification | Back Directory | [Name]
RITODRINE | [CAS]
26652-09-5 | [Synonyms]
Lavopa
Premar
ritodrina
RITODRINE
RITODRINE USP/EP/BP
Ritodrine Impurity 2 (Ritodrine)
(1R,2S)-2-(4-Hydroxyphenethylamino)-1-(4-hydroxyphenyl)-1-propanol
4-[(1R,2S)-1-Hydroxy-2-[2-(4-hydroxyphenyl)ethylamino]propyl]phenol
(1R,2S)-2-[(4-Hydroxyphenethyl)amino]-1-(4-hydroxyphenyl)-1-propanol
(1R,2S)-1-(4-Hydroxyphenyl)-2-[(4-hydroxyphenethyl)amino]-1-propanol
p-hydroxy-alpha-(1-((p-hydroxyphenethyl)amino)ethyl)-benzylalcohoerythr
(αR)-p-Hydroxy-α-[(S)-1-[(p-hydroxyphenethyl)amino]ethyl]benzyl alcohol
erythro-p-hydroxy-alpha-(1-((p-hydroxyphenethyl)amino)ethyl)benzylalcohol
4-hydroxy-alpha-(1-((2-(4-hydroxyphenyl)ethyl)amino)ethyl)-benzenemethano(
4-[2-[[(1S,2R)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]phenol
4-hydroxy-alpha-(1-((2-(4-hydroxyphenyl)ethyl)amino)ethyl)benzenemethanol(r*
(αR)-4-Hydroxy-α-[(S)-1-[[2-(4-hydroxyphenyl)ethyl]amino]ethyl]benzenemethanol
Benzyl alcohol, p-hydroxy-a-[1-[(p-hydroxyphenethyl)amino]ethyl]-, erythro- (8CI)
Benzenemethanol, 4-hydroxy-a-[1-[[2-(4-hydroxyphenyl)ethyl]amino]ethyl]-, (R*,S*)-
Benzenemethanol, 4-hydroxy-α-[(1R)-1-[[2-(4-hydroxyphenyl)ethyl]amino]ethyl]-, (αS)-rel-
Benzenemethanol, 4-hydroxy-a-[(1R)-1-[[2-(4-hydroxyphenyl)ethyl]amino]ethyl]-, (aS)-rel- (9CI) | [EINECS(EC#)]
247-879-9 | [Molecular Formula]
C17H21NO3 | [MDL Number]
MFCD00869731 | [MOL File]
26652-09-5.mol | [Molecular Weight]
287.35 |
| Hazard Information | Back Directory | [Description]
Ritodrine, 4-hydroxy-α-[1-[(4′-hydroxyphenethyl)amino]ethyl]benzylic alcohol
(11.1.19), differs slightly from epinephrine, and in the given example only one hydroxyl
group has been added to the aromatic ring of the phenylethylamino region of classic sympa�thomimetics. The second major difference between the examined series is the replacement of
the traditionally terminal iso-propyl or tert-butylamine region with a p-hydroxyphenylethy�lamine. Finally, the third difference is the presence of a methyl group at the α-atom of the
phenylethylamine region of sympathomimetics, which makes it similar to isoetharine. | [Uses]
Relaxant (smooth muscle). | [Uses]
Ritodrine is a selective β2-adrenoreceptor stimulant, predominantly of the urino-genital
system. It is used as a tocolytic agent for problems associated with premature miscar�riages, and only in specialized medical facilities. | [Definition]
ChEBI: (1S,2R)-ritodrine is a 4-[2-[[1-hydroxy-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]phenol that has (1S,2R)-configuration. It is an enantiomer of a (1R,2S)-ritodrine. | [General Description]
Ritodrine is a selective β2-agonist that wasdeveloped specifically for use as a uterine relaxant. Its uterineinhibitory effects are more sustained than its effects onthe cardiovascular system, which are minimal comparedwith those caused by nonselective β-agonists. The cardiovasculareffects usually associated with its administrationare mild tachycardia and slight diastolic pressure decrease.Usually, it is administered initially by intravenous infusionto stop premature labor and subsequently it may be givenorally. | [Clinical Use]
Ritodrine is a selective β2-agonist that is used exclusively for relaxing uterine muscle and inhibiting the contractions of premature labor.
Terbutaline, in addition to its use as a bronchodilator, also has been used for halting the contractions of premature labor. | [Synthesis]
Ritodrine is synthesized from 4-benzyloxypropiophenone, which undergoes bromination into
4-benzyloxy-|á-bromopropiophenone (11.1.17). This is reacted with 2-(4-benzy�loxyphenyl)ethylamine, forming an intermediate product (11.1.18), which undergoes
further debenzylation by hydrogen using a palladium catalyst, giving ritodrine (11.1.19)
[24,25].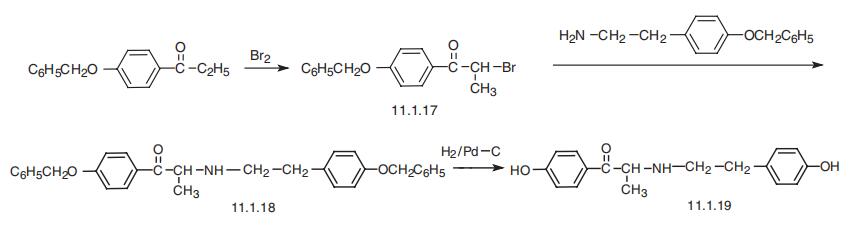
|
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
| Company Name: |
Roark Standards
|
| Tel: |
0755-83552066 15986688328 |
| Website: |
roarkstandards.com |
|