| Identification | Back Directory | [Name]
8-FLUOR-2-{4-[(METHYLAMINO)METHYL]FENYL}-1,3,4,5-TETRAHYDRO-6HAZEPINO[5,4,3-CD]INDOOL-6-ON | [CAS]
283173-50-2 | [Synonyms]
Rikapbu
AG014447
AG-14447
Rucaparib
AG 014447
PF-01367388
Rucaparib Base
AG 014447;AG014447
Rucaparib impurity
Rucaparib free base
Rucaparib(AG-014447)
rucaparib (PARP inhibitor) ORPHAN DRUG
AG-014699;PF-01367338; AG 014699;PF 01367338; AG014699;PF01367338
8-FLUOR-2-{4-[(METHYLAMINO)METHYL]FENYL}-1,3,4,5-TETRAHYDRO-6HAZEPINO[5,4,3-CD]INDOOL-6-ON
8-fluoro-2-(4-((methylamino)methyl)phenyl)-4,5-dihydro-1H-azepino[5,4,3-cd]indol-6(3H)-one
8-Fluoro-2-[4-[(methylamino)methyl]phenyl]-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one
8-fluoro-5-(4-((methylamino)methyl)phenyl)-2,3,4,6-tetrahydro-1H-azepino[5,4,3-cd]indol-1-one
8-Fluoro-1,3,4,5-tetrahydro-2-[4-[(methylamino)methyl]phenyl]-6H-pyrrolo[4,3,2-ef][2]benzazepin-6-one
6H-Pyrrolo[4,3,2-ef][2]benzazepin-6-one, 8-fluoro-1,3,4,5-tetrahydro-2-[4-[(methylamino)methyl]phenyl]- | [EINECS(EC#)]
814-445-0 | [Molecular Formula]
C19H18FN3O | [MDL Number]
MFCD11977252 | [MOL File]
283173-50-2.mol | [Molecular Weight]
323.36 |
| Chemical Properties | Back Directory | [Melting point ]
187 - 189°C | [Boiling point ]
625.2±55.0 °C(Predicted) | [density ]
1.281 | [storage temp. ]
Keep in dark place,Sealed in dry,2-8°C | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Yellow solid. | [pka]
14.10±0.20(Predicted) | [color ]
Pale Yellow to Yellow |
| Questions And Answer | Back Directory | [Description]
Rucaparib is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It was discovered as part of a collaboration between scientists working at the Northern Institute of Cancer Research and Medical School of Newcastle University and Agouron Pharmaceuticals in San Diego, California. It is being developed by Clovis Oncology.
| [Application]
Rucaparib is used to help maintain the response to other treatments for certain types of ovarian cancer (cancer that begins in the female reproductive organs where eggs are formed), fallopian tube (tube that transports eggs released by the ovaries to the uterus), and primary peritoneal (layer of tissue that lines the abdomen) cancer It is also used to treat certain types of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer in people with a specific gene who have not improved after treatment with at least two other therapies. Rucaparib is in a class of medications called poly (ADP-ribose) polymerase (PARP) inhibitors. It works by killing cancer cells.
| [Mechanism of action]
Rucaparib inhibits "the contraction of isolated vascular smooth muscle, including that from the tumours of cancer patients. It also reduces the migration of some cancer and normal cells in culture." As a PARP inhibitor, rucaparib is expected to be more effective in the 9% of pancreatic cancers with a BRCA mutation (BRCA1 or BRCA2).
|
| Hazard Information | Back Directory | [Uses]
Rucaparib is a poly (ADP ribose) polymerase (PARP) inhibitor. PARP is a DNA damage-activated nuclear enzyme that has a key signaling role in the base excision repair pathway. | [Definition]
ChEBI: Rucaparib is a member of the class of azepinoindoles that is 1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one carrying additional 4-[(methylamino)methyl]phenyl and fluoro substituents at positions 2 and 8 respectively. It is an inhibitor of poly (ADP-ribose) polymerase and is used (as the camsylate salt) as monotherapy for advanced ovarian cancer and deleterious germline or somatic BRCA mutation. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor and an antineoplastic agent. It is an azepinoindole, a member of caprolactams, an organofluorine compound and a secondary amino compound. It is a conjugate base of a rucaparib(1+). | [Synthesis]
Synthesis of rucaparib camsylate begins from commercially
available phthalimide acetal 151. Unveiling of
the aldehyde via treatment with aqueous HCl and precipitation
from toluene provided aldehyde 152 in 62% yield. To avoid
polymerization, 152 was immediately subjected to 6-fluoro-1Hindole-
4-carboxylic acid methyl ester (153) under reductive
conditions in the presence of acid to give rise to tryptamine
derivative 154. After considerable research, optimal conditions
for this transformation (triethylsilane in DCM/TFA) were
found that were successful on up to 15.7 kg scale, enabling
clean separation of the aldehyde reduction byproduct following
crystallization. Conversion of the phthalimide within 154 to
the corresponding amine using aqueous methylamine at room
temperature was accompanied by an intramolecular cyclization
reaction to secure the intermediate lactam as a solid in 89%
isolated yield. This was followed by a high-yielding bromination
reaction (83%) at the indole C-2 position employing
pyridinium tribromide, providing access to indoloazepinone
155. After screening various catalysts for the coupling of
bromide 155 and commercial boronic acid 156, Pd(dppf)Cl2?¤
DCM was found to reliably deliver the desired coupling
product with reasonable rates of reaction. Thus, after
development of an extensively optimized reaction protocol,
Suzuki coupling of 155 and 4-formylphenylboronic acid (156)
with Pd(dppf)Cl2?¤DCM and Na2CO3 in DMA at 90 ??C
generated the desired 2-arylated indole 157 in high yield (92%)
after trituration and reslurry with methanol. Conversion of
aldehyde 157 to amine 158 necessitated a two-pot procedure
designed to limit the formation of dimerization and aldehyde
reduction products that generally arise under conventional onepot
reductive amination conditions and have traditionally been
problematic on scale. Toward this end, subjection of aldehyde
157 to a methylamine solution in EtOH/MeOH/THF and
wash of the resulting reaction solids with methanol led to
efficient isolation of pure imine intermediate, which could be
immediately reduced with NaBH4 in THF/MeOH, providing
hydrochloride salt 158 upon acidic workup in 76% over two steps. The two remaining steps for conversion to the drug
involve a salt-swap, first breaking the HCl salt with NaOH in
MeOH, then treatment with (S)-camphorsulfonic acid/IPA/
H2O at 70 ??C. Filtration and washing of the cake with water
generated rucaparib camsylate (XVII) in 95% yield.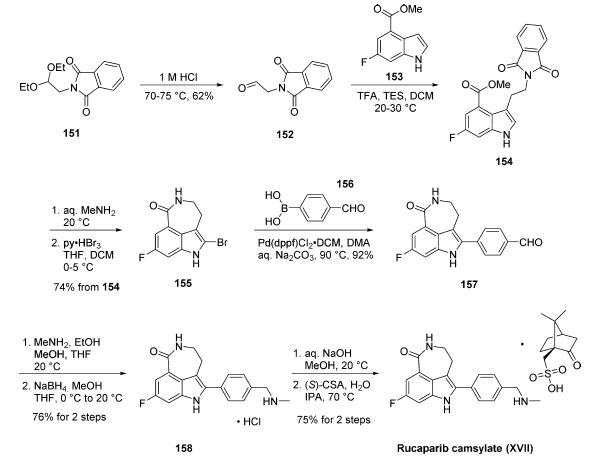
|
|
|