| Identification | Back Directory | [Name]
2-Oxiranecarboxylicacid, 3-(1,3-benzodioxol-5-yl)-2-Methyl-, ethyl ester | [CAS]
28578-16-7 | [Synonyms]
PMK BMK
new p 28578-16-7
BMK CAS 28578-16-7
PMK ethyl glycidate
3,4-MDP-2P ethyl ester
3,4-MDP-2-P intermediate
cheap 3,4-MDP-2P ethyl ester
3-(1,3-benzodioxol-5-yl)-2-Methyl-
3-(1,3-benzod.../3,4-MDP-2-P intermediate
Pharmaceutical intermediates 2-Oxiranecarboxylicacid
ethyl 3-(1,3-benzodioxol-5-yl)-2-methyloxirane-2-carboxylate
Ethyl 3-(1,3-benzodioxol-5-yl)-2-methyl-2-oxiranecarboxylate
ethyl 3-(benzo[d][1,3]dioxol-5-yl)-2-methyloxirane-2-carboxylate
2-Oxiranecarboxylicacid, 3-(1,3-benzod.../3,4-MDP-2-P intermediate
Ethyl 3-(1,3-benzodioxol-5-yl)-2-methyloxirane-2-carboxylate Liquid
2-Oxiranecarboxylicacid, 3-(1,3-benzodioxol-5-yl)-2-Methyl-, ethyl ester | [EINECS(EC#)]
234-232-0 | [Molecular Formula]
C13H14O5 | [MDL Number]
MFCD30478653 | [MOL File]
28578-16-7.mol | [Molecular Weight]
250.25 |
| Chemical Properties | Back Directory | [Boiling point ]
327.8±42.0 °C(Predicted) | [density ]
1.302±0.06 g/cm3(Predicted) | [solubility ]
DMF: 15 mg/ml; DMSO: 30 mg/ml; Ethanol: 10 mg/ml; PBS (pH 7.2): 1 mg/ml | [InChI]
InChI=1S/C13H14O5/c1-3-15-12(14)13(2)11(18-13)8-4-5-9-10(6-8)17-7-16-9/h4-6,11H,3,7H2,1-2H3 | [InChIKey]
BRILFEZHPXQINW-UHFFFAOYSA-N | [SMILES]
O1C(C2=CC=C3OCOC3=C2)C1(C)C(OCC)=O |
| Hazard Information | Back Directory | [Description]
PMK ethyl glycidate is an analytical reference standard categorized as a precursor in the synthesis of methylenedioxy phenethylamines and amphetamines, including 3,4-MDMA. This product is intended for research and forensic applications. | [Uses]
NSC 195099 can be used as an analytical reference standard. | [Application]
PMK methyl glycidate is applied as immediate precursors of 3,4-methylenedioxymethamphetamine. | [Synthesis]
To a solution of piperonal (1, 3.00 g, 19.9 mmol) in CH2Cl2 (100 mL) was added (carbethoxyethylidene)triphenylphosphorane (2,14.5 g, 40.0 mmol), and the reaction mixture was stirred for 24 h at room temperature. The mixture was then concentrated at reduced pressure, and the residue was purified by flash column chromatography on silica gel (hexanes/EtOAc,10:1) to afford olefin 3 (4.45 g, 95%) as a colorless oil: 1H NMR (400 MHz, CDCl3) d 7.58 (s, 1H), 6.92 (d, J = 1.7 Hz, 1H), 6.90 (dd, J = 7.9, 1.7 Hz, 1H), 6.82 (d, J = 8.0 Hz, 1H), 5.97 (s, 2H), 4.24 (q, J = 7.1 Hz, 2H), 2.10 (d, J = 1.6 Hz, 3H),1.33 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) d 168.7,147.6, 138.3, 129.9, 126.9, 124.6, 109.5, 108.2, 101.2, 60.7, 14.3,1 4.0; IR (ATR, cm-1): 2981, 2902,1698, 1628, 1502, 1489,1442, 1258, 1224; HRMS (EI) m/z calcd for C13H14O4 (M+) 234.0892, found 234.0895.
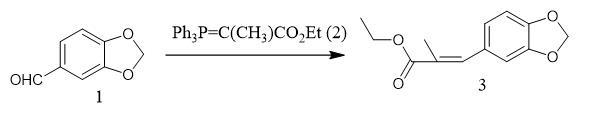 Fig The synthetic step of PMK ethyl glycidate Fig The synthetic step of PMK ethyl glycidate |
|