| Identification | Back Directory | [Name]
Doxapram | [CAS]
309-29-5 | [Synonyms]
ahr619
Dopram
dopream
AHR-619
DOXAPRAM
DOXOPRAM
4-(2-chloroethyl)-1-ethyl-3,3-diphenylpyrrolidin-2-one
1-Ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidone
1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidinon
1-Ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidinone
1-Ethyl-4-(2-morpholinoethyl)-3,3-diphenylpyrrolidin-2-one
2-Pyrrolidinone, 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-
1-ethyl-4-(2-(4-morpholinyl)ethyl)-3,3-diphenyl-2-pyrrolidinon
1-Ethyl-4-[2-(4-morpholinyl)ethyl]-3,3-diphenyl-2-pyrrolidinone
1-Ethyl-4-(2-morpholin-4-ylethyl)-3,3-di(phenyl)pyrrolidin-2-one
2-Pyrrolidinone, 1-ethyl-4-[2-(4-morpholinyl)ethyl]-3,3-diphenyl-
2-Pyrrolidinone, 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl- (7CI, 8CI) | [EINECS(EC#)]
206-216-3 | [Molecular Formula]
C24H30N2O2 | [MDL Number]
MFCD00242721 | [MOL File]
309-29-5.mol | [Molecular Weight]
378.51 |
| Hazard Information | Back Directory | [Uses]
Doxapram HCl inhibits TASK-1, TASK-3, TASK-1/TASK-3 heterodimeric channel function with EC50 of 410 nM, 37 μM, 9 μM, respectively See more at: http://www.selleckchem.com/products/doxapram-hcl.html#sthash.SSt04Hwr.dpuf | [Definition]
ChEBI: A member of the class of pyrrolidin-2-ones that is N-ethylpyrrolidin-2-one in which both of the hydrogens at the 3 position (adjacent to the carbonyl group) are substituted by phenyl groups, and one of the hydrogens at the 4 position is
ubstituted by a 2-(morpholin-4-yl)ethyl group. A central and respiratory stimulant with a brief duration of action, it is used (generally as the hydrochloride or the hydrochloride hydrate) as a temporary treatment of acute respiratory failure, particularly
when superimposed on chronic obstructive pulmonary disease, and of postoperative respiratory depression. It has also been used for treatment of postoperative shivering. | [Originator]
Dopram,Robins,US,1965 | [Manufacturing Process]
(A) Preparation of α-(1-ethyl-3-pyrrolidyl)-α,α-diphenylacetonitrile: A
suspension of the sodium salt of diphenylacetonitrile was formed by the
dropwise addition at 50°C of 193 grams (1.0 mol) of diphenylacetonitrile to a
stirred suspension of 43 grams (1.1 mols) of sodium amide in 1 liter of dry
toluene. After addition was complete, the mixture was refluxed for 4 hours
and then, to the refluxing mixture, 1.0 mol of 1-ethyl-3-chloropyrrolidine was
added at a rapid dropwise rate with continuous stirring. After addition was
complete, stirring and refluxing were continued for 3 hours. The mixture was
then cooled and extracted with one normal hydrochloric acid. The aqueous
layer together with an oil layer were separated, made basic with dilute sodium
hydroxide, and extracted with ether. The ethereal solution was dried over
sodium sulfate and concentrated and the residue was distilled in vacuo. The
material crystallized from a 4:1 ethanol-water mixture.
(B) Preparation of 4-(β-chloroethyl)-3,3-diphenyl-1-ethyl-2-pyrrolidinone: A
solution of α,α-diphenyl-α-(1-ethyl-3-pyrrolidyl)-acetonitrile in 70% sulfuric
acid was heated at 130-140°C for 48 hours, poured onto ice, made basic with
sodium hydroxide, and extracted with chloroform. The chloroform solution was
acidified with hydrogen chloride gas, dried over sodium sulfate and
concentrated. The residue was refluxed in 500 ml of thionyl chloride for 3
hours; the resulting solution was concentrated in vacuo; and the residue wascrystallized from isopropyl ether.
(C) Preparation of doxapram hydrochloride [3,3-diphenyl-1-ethyl-4-(2-
morpholino-ethyl)-2-pyrrolidinone hydrochloride monohydrate]: A solution of
25 grams (0.076 mol) of 4-(2-chloroethyl)-3,3-diphenyl-1-ethyl-2-
pyrrolidinone and 13.3 grams (0.153 mol) of morpholine in 500 ml of absolute
ethanol was heated at 95°-120°C for 21 hours in a closed system and
concentrated in vacuo. The residue was dissolved in 300 ml of two normal
hydrochloric acid and extracted with 150 ml of ethyl acetate. A solid
crystallized (13 g) during the extraction and was removed by filtration. MP
217°-219°C. The acid extracts were made basic with sodium hydroxide and
extracted with ether, and the ether solution was concentrated in vacuo and
the residue was suspended in six normal hydrochloric acid. Additional
crystalline product formed and was recrystallized from two normal
hydrochloric acid. Yield, 10 grams; MP 217°-219°C. Total yield, 23 grams
(70%). | [Therapeutic Function]
Respiratory stimulant | [Synthesis]
Doxapram, 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidinone
(8.2.4), is synthesized in the following manner. Diphenylacetonitrile in the presence of
sodium amide is alkylated with 1-ethyl-3-chlorpyrrolidine, giving (1-ethyl-3-pyrrolidinyl)
diphenylacetonitrile (8.2.1). Acidic hydrolysis of the nitrile group gives (1-ethyl-3
pyrrolidinyl)diphenylacetic acid (8.2.2). Reacting this with phosphorous tribromide (thionyl chloride, thionyl bromide, acetic anhydride) leads to rearrangement with an open�ing of the pyrrolidine ring and the subsequent closing of the pyrrolidinone ring, forming
1-ethyl-4-(2-bromoethyl)-3,3-diphenyl-2-pyrrolidinone (8.2.3). Substitution of the
bromine atom with a morpholine group gives doxapram (8.2.4) [15¨C18].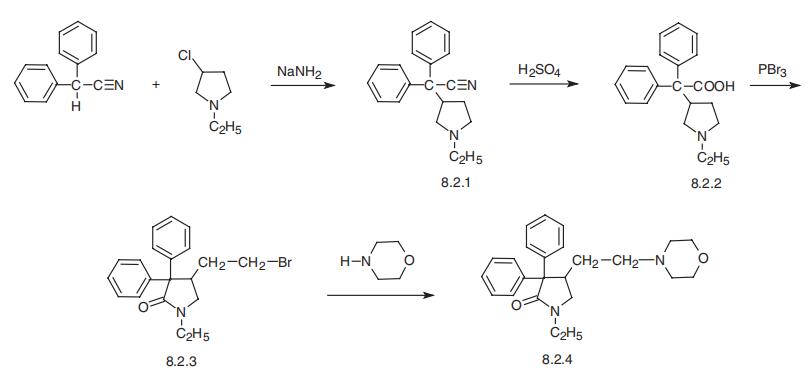
|
|
|