| Identification | Back Directory | [Name]
O-ALLYLHYDROXYLAMINE HYDROCHLORIDE | [CAS]
38945-21-0 | [Synonyms]
(Allyloxy)amine hydrochloride
O-ALLYHYDROXYAMINE HYDROCHLORIDE)
O-ALLYLHYDROXYLAMINE HYDROCHLORIDE
3-(Aminooxy)prop-1-ene hydrochloride
O-prop-2-enylhydroxylamine hydrochloride
O-(2-PROPENYL)HYDROXYLAMINE HYDROCHLORIDE
O-Allylhydroxylamine Hydrochloride
HydroxylaMine, O-2-propenyl-, hydrochloride
O-Allylhydroxylamine hydrochloride >=98.0% (AT)
Hydroxylamine, O-2-propenyl-, hydrochloride (9CI) | [EINECS(EC#)]
254-203-6 | [Molecular Formula]
C3H8ClNO | [MDL Number]
MFCD00012957 | [MOL File]
38945-21-0.mol | [Molecular Weight]
109.55 |
| Chemical Properties | Back Directory | [Melting point ]
~170 °C (dec.)
| [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [form ]
powder to crystal | [color ]
White to Almost white | [BRN ]
3552394 | [InChI]
InChI=1S/C3H7NO.ClH/c1-2-3-5-4;/h2H,1,3-4H2;1H | [InChIKey]
XIQUJVRFXPBMHS-UHFFFAOYSA-N | [SMILES]
C(ON)C=C.Cl |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
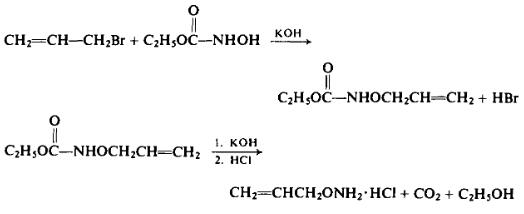
To a solution of 86.8 gm (1.55 moles) of potassium hydroxide in 330 ml of absolute ethanol is added a solution of 159 gm (1.52 moles) of ethyl N-hydroxycarbamate in 330 ml of absolute ethanol. With external cooling to maintain an internal temperature of 25°C to this mixture is added 195.5 gm (1.62 moles) of allyl bromide. After the addition has been completed, the mixture is heated under reflux for 2 hr. After separating the potassium bromide formed during the reaction and washing it with absolute alcohol, the alcoholic solution is evaporated under reduced pressure. The residue is dissolved in ether and then the ether solution is extracted repeatedly with 10% aqueous sodium hydroxide solution. [From the ether solution, on evaporation 25.5 gm (18%) of ethyl N,O-diallylhydroxycar-bamate, b.p. 91-92°C (8.55 mm Hg) may be isolated.] The aqueous extract is acidified with 10% aqueous sulfuric acid and the ethyl O-allylhy-droxycarbamate is extracted with ether. Upon evaporating the ether off, 134.2 gm (61%) of the intermediate product, b.p. 107°C (12.5 mm Hg), is isolated.
In a steam distillation apparatus, 134 gm of ethyl O-allylhydroxy-carbamate is treated with a solution of 120 gm of potassium hydroxide in 280 ml of water. The product is steam-distilled into a receiver containing dilute hydrochloric acid.
The steam distillate is evaporated under reduced pressure. The residue is taken up twice in absolute ethanol and dried by evaporation under reduced pressure. The yield is 91 gm (55%, overall), m.p. 169-170°C. Upon recrystallization from absolute alcohol and dry ether, the melting point is raised to 170.6-170.8°C (172-174°C). Free O-allylhydrox-ylamine has the following reported properties: b.p. 98-99°C, n25D 1.4300.
The problems connected with the preparation of O-arylhydroxylamines by this method have been attributed to the instability of the aryl- substituted aminooxy group to the hydrolytic system used in its preparation. This problem has recently been circumvented by substituting for ethyl N-hydroxycarbamate, t-butyl N-hydroxycarbamate.
|
| Hazard Information | Back Directory | [References]
[1] Patent: US2014/378399, 2014, A1. Location in patent: Paragraph 0094
[2] Journal of Pharmacology and Experimental Therapeutics, 1999, vol. 288, # 2, p. 490 - 501
[3] European Journal of Medicinal Chemistry, 2014, vol. 75, p. 184 - 194
[4] Synlett, 2017, vol. 28, # 2, p. 214 - 220 |
|
|