| Identification | Back Directory | [Name]
R(+)-PROPRANOLOL HCL | [CAS]
5051-22-9 | [Synonyms]
(+)-2-propano
(r)-2-propano
d-propranolol
dexpropranolol
2r-propranolol
(+)-propranolol
d-(+)-propranolol
dextropropranolol
R(+)-PROPRANOLOL HCL
R(+)-PROPRANOLOL HCL USP/EP/BP
R(+)-PROPRANOLOL HYDROCHLORIDE >98%
(R)-1-Isopropylamino-3-(1-naphtyloxy)-2-propanol
(2R)-1-(Isopropylamino)-3-(1-naphtyloxy)propane-2-ol
(2R)-1-(isopropylamino)-3-(1-naphthyloxy)propan-2-ol
(2R)-1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol
2-Propanol, 1-(isopropylamino)-3-(1-naphthyloxy)-, (+)- (8CI)
2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, (R)-
2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, (2R)-
2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, (2R)- (9CI)
(R)-(+)-1-(1-Methylethylamino)-3-naphthalen-1-yloxy-propan-2-ol hydrochloride | [EINECS(EC#)]
225-749-2 | [Molecular Formula]
C16H21NO2 | [MDL Number]
MFCD00066275 | [MOL File]
5051-22-9.mol | [Molecular Weight]
259.34 |
| Chemical Properties | Back Directory | [Melting point ]
96 °C | [Boiling point ]
434.9±30.0 °C(Predicted) | [density ]
1.093±0.06 g/cm3(Predicted) | [storage temp. ]
Store at RT | [pka]
pKa 9.53±0.01(H2O,t=25±0.5,I=0.15(KCl))(Approximate) |
| Hazard Information | Back Directory | [History]
Scottish scientist James W. Black developed propranolol in the 1960s. It was the first beta-blocker effectively used in the treatment of coronary artery disease and hypertension.
Propranolol was patented in 1962 and approved for medical use in 1964. It is on the World Health Organization's List of Essential Medicines. In 2023, it was the 69th most commonly prescribed medication in the United States, with more than 9 million prescriptions. | [Uses]
Propranolol has been studied most carefully in experiments and in clinics. It is used for
ventricular tachycardia, arrhythmia caused by digitalis drug overdose, or as a result of thyrotoxosis
or excess catecholamine activity. Despite the fact that there are a number of
β-adrenoblockers, propranolol is considered the first choice of drugs although other blockers
of calcium blockers can be just as effective. | [Uses]
Propranolol is used for treating hypertension, angina pectoris, supraventricular arrhythmia,
ventricular tachycardia, migraines, hypertrophic subaortal stenosis, and pheochromocytosis.
It is used following a myocardial infraction. | [Uses]
Propranolol is used in treating arterial hypertonicity, angina, extrasystole, superventric�ular arrhythmia, ventricular tachycardia, migraines, hypertrophic subaortic stenosis, and
pheochromocytoma. It also is used in the postanginal phase of myocardial infarctions. | [Definition]
ChEBI: (R)-(+)-propranolol is a propranolol. | [Indications]
Propranolol slows heart rate, increases the effective refractory period of atrioventricular
ganglia, suppresses automatism of heart cells, and reduces excitability and contractibility
of the myocardium. It is used for supraventricular and ventricular arrhythmias. | [Synthesis Reference(s)]
Tetrahedron Letters, 31, p. 2157, 1990 DOI: 10.1016/0040-4039(90)80097-6 | [General Description]
R(+)-PROPRANOLOL HCL(Inderal, others) is the prototypical andnonselective β-blocker. It blocks the β1- and β2-receptorswith equal affinity, lacks ISA, and does not block β-receptors. R(+)-PROPRANOLOL HCL like the other β-blockers discussed,is a competitive blocker whose receptor-blockingactions can be reversed with sufficient concentrations of β-agonists.
| [Biological Activity]
Less active enantiomer of the β -adrenoceptor antagonist propranolol ((RS)-1-[(1-Methylethyl)amino]-3-(1-naphthalenyloxy)-2-propanol hydrochloride ). | [Mechanism of action]
Propranolol is a nonselective β-adrenoblocker that affects both the mechanical and electrophysiological
properties of the myocardium. It lowers myocardial contractibility, heart
rate, blood pressure, and the myocardial need for oxygen. These properties make propranolol
and other β-adrenoblockers useful antianginal drugs. | [Clinical Use]
Currently, R(+)-PROPRANOLOL HCL is approved for use inthe United States for hypertension, cardiac arrhythmias,angina pectoris, postmyocardial infarction, hypertrophiccardiomyopathy, pheochromocytoma, migraine prophylaxis,and essential tremor. In addition, because of its highlipophilicity (log P=3.10) and thus its ability to penetratethe CNS, propranolol has found use in treating anxiety andis under investigation for the treatment of a variety of otherconditions, including schizophrenia, alcohol withdrawalsyndrome, and aggressive behavior.
| [Side effects]
The toxicity associated with propranolol is for the most
part related to its primary pharmacological action, inhibition
of the cardiac β-adrenoceptors. In addition, propranolol
exerts direct cardiac depressant effects that become
manifest when the drug is administered rapidly by the
IV route.Glucagon immediately reverses all cardiac depressant
effects of propranolol, and its use is associated
with a minimum of side effects. The inotropic agents
amrinone (Inocor) and milrinone (Primacor) provide
alternative means of augmenting cardiac contractile
function in the presence of β-adrenoceptor blockade. Propranolol may also stimulate bronchospasm in patients with asthma.
Since propranolol crosses the placenta and enters the
fetal circulation, fetal cardiac responses to the stresses
of labor and delivery will be blocked. Additionally,
propranolol crosses the blood-brain barrier and is associated
with mood changes and depression. School difficulties
are commonly associated with its use in children.
Propranolol may also cause hypoglycemia in infants. | [Synthesis]
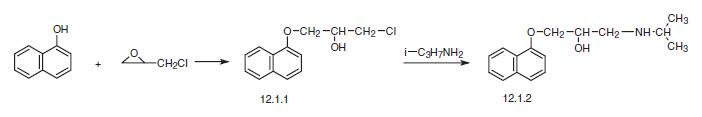
Propranolol, 1-(iso-propylamino)-3-(1-naphthyloxy)-2-propanol (12.1.2), is synthesized in two ways from the same initial substance. The first way consists of reacting 1-naphthol with epichlorohydrin. Opening of the epoxide ring gives 1-chloro-3- (1-naphthyloxy)-2-propanol (12.1.1), which is reacted further with iso-propylamine, giving propranolol (12.1.2).
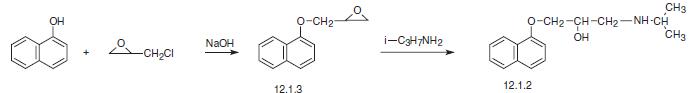
The second method uses the same reagents in the presence of a base and consists of ini�tially making 3-(1-naphthyloxy)propylenoxide (12.1.3), the subsequent reaction with iso�propylamine which results in epoxide ring opening leading to the formation of propranolol (12.1.2) [1–6].
| [Metabolism]
Propranolol (Inderal) is suitable for both parental and
oral administration. Absorption from the gastrointestinal
tract is extensive. The peak therapeutic effect after
oral administration occurs in 1 to 1.5 hours.The plasma
half-life of propranolol is approximately 3 hours. The
drug is concentrated in the lungs and to a lesser extent
in the liver, brain, kidneys, and heart. Binding to plasma
proteins is extensive (90%). The liver is the chief organ
involved in the metabolism of propranolol, and the drug
is subject to a significant degree of first-pass metabolism.
At least eight metabolites have been recovered
from the urine, the major excretory route. | [Precautions]
Propranolol is contraindicated for patients with depressed
myocardial function and may be contraindicated in the presence of digitalis toxicity because of the possibility
of producing complete A-V block and ventricular
asystole. Patients receiving anesthetic agents that tend to
depress myocardial contractility (ether, halothane)
should not receive propranolol. Propranolol should be
used with extreme caution in patients with asthma.
Up-regulation of β-receptors follows long-term
therapy, making abrupt withdrawal of β-blockers dangerous
for patients with ischemic heart disease. |
|
| Company Name: |
Enzo Biochem Inc
|
| Tel: |
Enzo Biochem Inc. 13797054060 |
| Website: |
www.enzo.com |
|