| Identification | Back Directory | [Name]
Nalmefene | [CAS]
55096-26-9 | [Synonyms]
C08027
ORF-11676
NALMEFENE
nalmetrene
Nalmefene d3
Nalmefene-D7
NalMefene USP
6-Deoxo-6-methylenenaltrexone
6-desoxy-6-methylenenaltrexone
17-(Cyclopropylmethyl)-4,5α-epoxy-6-methylenemorphinan-3,14-diol
4,5α-Epoxy-6-methylene-17-(cyclopropylmethyl)morphinan-3,14-diol
9a-(Cyclopropylmethyl)-4,5alpha-epoxy-6-methylen-3,14-morphinandiol
(5α)-17-(cyclopropylmethyl)-4,5-epoxy-6-methylenemorphinan-3,14-diol
17-(cyclopropylmethyl)-4,5alpha-epoxy-6-methylenemorphinan-3,14-diol
(5α)-17-(d3-Cyclopropylmethyl)-4,5-epoxy-6-methylenemorphinan-3,14-diol
Morphinan-3,14-diol,17-(cyclopropylmethyl)-4,5-epoxy-6-methylene-, (5a)-
[5ALPHA]-17-[CYCLOPROPYLMETHYL]-4,5-EPOXY-6-METHYLENEMORPHINAN-3,14-DIOL
(5alpha)-17-(cyclopropylmethyl)-4,5-epoxy-6-methylenemorphinon-3,14-diol
Morphinan-3,14-diol, 17-(cyclopropylmethyl)-4,5-epoxy-6-methylene-, (5α)-
Nalmefene-d3Q: What is
Nalmefene-d3 Q: What is the CAS Number of
Nalmefene-d3
(4R,4aS,7aS,12bS)-3-(cyclopropylmethyl)-7-methylene-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,9-diol | [Molecular Formula]
C21H25NO3 | [MDL Number]
MFCD00133650 | [MOL File]
55096-26-9.mol | [Molecular Weight]
339.43 |
| Chemical Properties | Back Directory | [Melting point ]
182-185?C | [Boiling point ]
507.9±50.0 °C(Predicted) | [density ]
1.38±0.1 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
Chloroform (Slightly), Dichloromethane (Slightly), DMSO (Slightly) | [form ]
Solid | [pka]
pKa 7.63(H2O) (Uncertain) | [color ]
White to Off-White | [CAS DataBase Reference]
55096-26-9 | [NIST Chemistry Reference]
Nalmefene(55096-26-9) |
| Hazard Information | Back Directory | [Description]
Nalmefene is a 6-methylene analogue of naltrexone.
It is a pure opioid receptor antagonist
and has a high affinity for the κ-opioid receptor.
Nalmefene has a long duration of action and is
used in oral and parenteral formulations. | [Chemical Properties]
Off-White Solid | [Originator]
Nalmefene,Mallinckrodt Inc. | [Uses]
A structural analog of Naltrexone (N285780) with opiate antagonist activity used in pharmaceutical treatment of alcoholism. Other pharmacological applications of this compound aim to reduce food cravings, drug abuse and pulmonary disease in affected individuals. Used as an opioid-induced tranquilizer on large animals in the veterinary industry. Narcotic antagonist. | [Uses]
A structural labelled analog of Naltrexone (N285780) with opiate antagonist activity used in pharmaceutical treatment of alcoholism. Other pharmacological applications of this compound aim to reduce f
ood cravings, drug abuse and pulmonary disease in affected individuals. Used as an opioid-induced tranquilizer on large animals in the veterinary industry. Narcotic antagonist. | [Uses]
opioid antagonists therapeutic for alcohol dependence | [Definition]
ChEBI: Nalmefene is a morphinane alkaloid. | [Manufacturing Process]
A dry, 2-liter, 3-neck, round bottom flask fitted with two stoppers and a
magnetic stirring bar was charged with potassium t-butoxide (61.1 g, 0.545
mol) and methyltriphenylphosphonium bromide (194.4 g, 0.544 mol). Freshly
distilled tetrahydrofuran (450 ml) was introduced at 20°C. The resultant thick,
bright yellow dispersion was stirred at 20°C for 0.5 h and further dry
tetrahydrofuran (100 ml) was added. A solution of dry naltrexone (30 g, 0.088
mol) in dry tetrahydrofuran (200 ml) was then added dropwise over 40 min.
Then the reaction mixture was stirred for a further 1.25 h, then cooled to
10°C, and quenched with 20% aqueous ammonium chloride solution (75 ml)
followed by water (100 ml). The organic layer was separated and the aqueous
layer extracted with four 100 ml portions of chloroform. Solvent was
evaporated from the tetrahydrofuran layer and the combined chloroform
extracts, the residues combined and brought to pH 2 by addition of 2 N
hydrochloric acid. The resultant precipitate was filtered, washed with
chloroform and suspended in a mixture of chloroform (500 ml) and water
(250 ml). Ammonium hydroxide was added to attain a pH of 8 and the
aqueous layer separated. The organic layer was dried over anhydrous sodium
sulfate, filtered, and the solvent removed in vacuo. The resultant solid was
dissolved in ethyl acetate (1400 ml), the solution filtered through a silica pad
and the solvent evaporated. The product was recrystallized from chloroform
and washed with hexane to yield pure 6-desoxy-6-methylenenaltrexone (also
called nalmefene) as a white solid. Yield: 27.0 g, 88%. | [Therapeutic Function]
Antagonist to narcotics | [Biological Functions]
Nalmefene (Revex) is a long-acting injectable pure opioid
antagonist recently introduced in the United States.
It binds all opioid receptors and reverses the effects of
opioid agonists at those receptors.The onset of action is 2 minutes after IV administration. Hepatic metabolism
is slow and occurs via glucuronide conjugation to inactive
metabolites. Its half-life of 11 hours is about 5 times
that of naloxone. Indications include use in postoperative
settings to reverse respiratory depression and in
opioid overdose. Due to the long duration of action of
nalmefene, however, naloxone may be preferred for
treatment of overdose because it produces a shorter duration
of withdrawal effects. | [General Description]
Nalmefene (Revex) is a pure opioid antagonist that is the6-methylene analog of naltrexone. It is available as a solutionfor IV, IM, or subcutaneous (SC) administration toreverse the effects of opioids after general anesthesia andin the treatment of overdose. It is longer acting than naloxonebut otherwise has a similar pharmacodynamic andmetabolic (3-glucuronidation) profile. Nalmefene hashigher oral bioavailability (approximately 40%) thannaloxone or naltrexone and is currently being investigatedas an oral treatment for pathological gambling and alcoholabuse. | [Biochem/physiol Actions]
Nonselective opioid receptor antagonist. | [Synthesis]
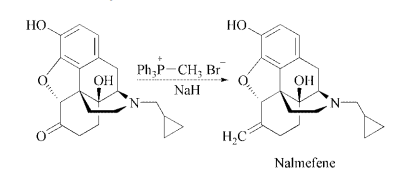
Nalmefene is synthesized by a
Wittig reaction of naltrexone with triphenylmethylphosphonium
bromide in DMSO under
basic catalysis of NaH.
 |
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
| Company Name: |
TOSUN PHARM
|
| Tel: |
020-61855200 13326451905 |
| Website: |
www.toref.cn/ |
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|