| Identification | Back Directory | [Name]
BRADYKININ | [CAS]
58-82-2 | [Synonyms]
bk
brs640
prs640
C00306
Callideic
KallidinⅠ
BRADYZIDE
RPPGFSPFR
BRADYKININ
Callidin I
Kallidin 9
Kallidin I
Bradykinin, >98%
BRADYKININ, LYSYL
BRADYKININ, HUMAN
BRADYKININ, TYROSYL
Synthetic bradykinin
bradykinin(synthetic)
BRADYKININ, METHIONYL-LYSYL
ARG-PRO-PRO-GLY-PHE-SER-PRO-PHE-ARG
Bradykinin - CAS 58-82-2 - Calbiochem
BRADYKININ (HUMAN, BOVINE, RAT, MOUSE)
Leucine Enkephalin acetate salt hydrate
H-ARG-PRO-PRO-GLY-PHE-SER-PRO-PHE-ARG-OH | [EINECS(EC#)]
200-398-8 | [Molecular Formula]
C50H73N15O11 | [MDL Number]
MFCD00076258 | [MOL File]
58-82-2.mol | [Molecular Weight]
1060.21 |
| Chemical Properties | Back Directory | [Appearance]
Amorphous solid. | [Melting point ]
170°C (rough estimate) | [alpha ]
D25 -76.5° (c = 1.37 in 1N acetic acid) | [Boiling point ]
811.85°C (rough estimate) | [density ]
1.1171 (rough estimate) | [refractive index ]
1.6930 (estimate) | [RTECS ]
EE1530000 | [storage temp. ]
−20°C
| [solubility ]
H2O: >40 mg/mL
| [form ]
White to off-white lyophilized solid | [pka]
3.34±0.10(Predicted) | [color ]
Lyophilized powder | [Water Solubility ]
Soluble to 1 mg/ml in water. | [Sequence]
H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH | [CAS DataBase Reference]
58-82-2 |
| Hazard Information | Back Directory | [Chemical Properties]
Amorphous solid. | [Definition]
ChEBI: A linear nonapeptide messenger belonging to the kinin group of proteins, with amino acid sequence RPPGFSPFR. Enzymatically produced from kallidin in the blood, it is a powerful vasodilator that causes smooth muscle contraction, and may mediate inflammation | [Hazard]
Powerful vasodilator; increased capillary
permeability; stimulates pain receptors; contraction
of smooth muscle; teratogen; mutagenic.
| [Uses]
Bradykinin is used to lower blood pressure, increase bradykinin (by inhibiting its degradation) further lowering blood pressure. Bradykinin dilates blood vessels via the release of prostacyclin, nitric oxide, and endothelium-derived hyperpolarizing factor. | [Uses]
Leucine Enkephalin acetate salt hydrate has been used in the calibration of the Q-Tof Micro hybrid, triple quadrupole-orthogonal acceleration time of flight (TOF) instrument in single-stage TOF mode. It has also been used to perform the calibration of mass spectrometry. It has also been used in liquid chromatography-mass spectrometry (LCMS)/MS for calibration of the system and for relative correction of the spectra. | [Biological Functions]
The kallikrein–kinin system is an enzymatic pathway
giving rise to two predominant vasoactive peptides,
kallidin and bradykinin. Kallikrein, the enzyme responsible
for the formation of these peptides, exists in
plasma and tissues. However, circulating levels of the
end products, kallidin and bradykinin, are quite low because
the kallikrein enzymes are present largely in inactive
forms. In addition, the short half-life of these peptides
(15 seconds) also contributes to low plasma levels.
In general, the kinins produce relaxation of vascular
smooth muscle and vasodilation. Bradykinin causes vascular smooth muscle relaxation by stimulating the
endothelium to release prostacyclin and nitric oxide.
Blood flow to the brain, heart, viscera, skeletal muscle,
and glands is increased. In nonvascular smooth muscle,
bradykinin will produce a contractile response.
Other actions of kinins include activation of clotting
factors simultaneously with the production of bradykinin.
In the kidney, bradykinin production results in an
increase in renal papillary blood flow, with a secondary
inhibition of sodium reabsorption in the distal tubule. In
the peripheral nervous system, bradykinin is important
for the initiation of pain signals. It is also associated with
the edema, erythema, and fever of inflammation.
Bradykinin exerts its physiological effects via two
receptors, the B1 and B2 receptors, with most of its
physiological effects being mediated by the B2 receptor.
The precise function of the B1 receptor is unclear;
however, some of the chronic inflammatory responses
to bradykinin may be mediated through actions at this
receptor.
Bradykinin antagonists of the B2 receptor are currently
in development and may find utility in the treatment
of pain associated with burns and such chronic inflammatory
disorders as arthritis, asthma, and chronic
pain. | [General Description]
Induces the release of nitric oxide. Other physiological functions include stimulation of pain receptors, inhibition of cAMP accumulation, induction of smooth muscle contraction, and vasodilation. Also involved in edema resulting from trauma or injury. Improves post-ischemic recovery of heart via a nitric oxide-dependent mechanism. | [Biochem/physiol Actions]
Product does not compete with ATP. | [Clinical Use]
9-peptide, produced in response to tissue
damage, inflammation, viral infections,
etc. Produces pain, increased vascular
permeability, and synthesis of
prostaglandins. | [storage]
Store at -20°C |
| Questions And Answer | Back Directory | [Receptors]
BKs signal via two GPCRs known as the B1 (BDKRB1)
and B2 (BDKRB2) receptors. These BK receptor genes
were the result of tandem duplication that predated the
teleost tetraploidization while no extra copy was found
in teleosts. B2 is constitutively expressed and predominant in different tissues while B1 expression is induced
by inflammation. B1 expression in inflammatory tissues
such as a wound area and vascular injury contributes
to the inflammatory edema by its vasodilatory effect on
local blood vessels. B2 is ubiquitously expressed in different tissues. In the aorta and large muscular arteries,
including the carotid and mesenteric arteries, B2 is localized predominantly in the endothelium. However, in
small arterioles toward the urinary bladder, myometrium, breast, etc., B2 is located predominantly in the
smooth muscle rather than the endothelium. bdkrb2-
overexpressed zebrafish induce IP3 accumulation by BK
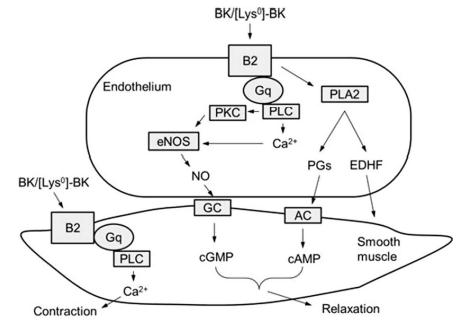
with an EC50 of 6.6 nM. | [Signal transduction pathway]
Both B1 and B2 trigger a typical Ca signaling of
GPCRs. Receptor activation leads to phospholipase
C (PLC) activation, intracellular Ca mobilization, release
of endothelium-dependent relaxation factor (EDRF),
and activation of PKC and cytosolic PLA2 that results
in the release of prostaglandins (PGs). The endothelial
nitric oxide synthase (eNOS) is also stimulated by B2
activation, leading to an increase in NO, stimulation
of guanylyl cyclase, and an increase in cGMP. Most of
these factors are EDRFs and are contributing to the
hypotensive effect of BK. However, in smooth muscle
cells, B2 activation activates PLC directly, leading to a
transient increase in Ca2+ flux to induce muscle contraction. Although BK mostly induces vasorelaxation via
endothelium-dependent signalings, in small arterioles
where B2 is predominately localized in the smooth
muscle cells, BK induces vasoconstriction.
In the light of this dual function, [Arg0
]-BK injection
in teleosts was a vasopressor and could be related to
a direct stimulation of B2 on smooth muscles, as the
endothelium-dependent relaxing system is not prominent in teleosts.
 | [Agonists and Antagonists]
[Lys0
, des-Arg9
]-BK is used as a B1 receptor agonist.
Cereport (RMP-7) is a selective B2 agonist, and has been
shown to transiently increase the permeability of the
blood-brain barrier. The B1 antagonist BI-113823 possesses an antiinflammatory function. Icatibant (HOE 140 or JE 049) is a potent,
specific, and selective peptidomimetic B2 antagonist. |
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|