| Identification | Back Directory | [Name]
OPC-67683 | [CAS]
681492-22-8 | [Synonyms]
CS-2255
OPC-67683
Delamanid
Delamanid (OPC-67683)
DELAMANID;OPC67683;OPC 67683
(2R)-2-METHYL-6-NITRO-2-[[4-[4-[4-(TRIFLUOROMETHOXY)PHENOXY]PIPERIDIN-1-YL]PHENOXY]METHYL]-3H-IMIDAZO[2,1-B][1,3]OXAZOLE
(R)-2-Methyl-6-nitro-2-((4-(4-(4-(trifluoromethoxy)phenoxy)piperidin-1-yl)phenoxy)methyl)-2,3-dihydroimidazo[2,1-b]oxazole
(2R)-2,3-Dihydro-2-methyl-6-nitro-2-[[4-[4-[4-(trifluoromethoxy)phenoxy]-1-piperidinyl]phenoxy]methyl]imidazo[2,1-b]oxazole
Imidazo[2,1-b]oxazole, 2,3-dihydro-2-methyl-6-nitro-2-[[4-[4-[4-(trifluoromethoxy)phenoxy]-1-piperidinyl]phenoxy]methyl]-, (2R)- | [Molecular Formula]
C25H25F3N4O6 | [MDL Number]
MFCD18251539 | [MOL File]
681492-22-8.mol | [Molecular Weight]
534.48 |
| Chemical Properties | Back Directory | [Melting point ]
195-196℃ | [Boiling point ]
653.7±65.0 °C(Predicted) | [density ]
1.45 | [storage temp. ]
Hygroscopic, -20°C Freezer, Under inert atmosphere | [solubility ]
Chloroform (Sparingly), Ethyl Acetate (Slightly, Sonicated) | [form ]
Solid | [pka]
3.99±0.20(Predicted) | [color ]
Off-White to Light Yellow | [Stability:]
Hygroscopic |
| Hazard Information | Back Directory | [Description]
Marketed by Otsuka, delamanid was approved in both the European
Union and Japan in 2014 as part of combination therapies for
multi-drug resistant tuberculosis (TB). Because delamanid exhibited
no adverse drug–drug interactions, it has found utility as a
combination therapy with standard antiretroviral drugs indicated
for TB. Delamanid blocks mycolic acid biosynthesis in Mycobacterium
tuberculosis, which allows its cell wall to be penetrated by
small molecule antivirals. | [Uses]
Delamanid is a novel anti-tuberculosis medication that inhibits mycolic acid synthesis and shows potent in vitro and in vivo activity against drug-resistant strains of Mycobacterium tuberculosis. | [Definition]
ChEBI: Delamanid is a member of piperidines. | [Clinical Use]
Treatment of multi-drug resistant tuberculosis | [Synthesis]
Piperidine 81 was concurrently prepared by first generating
biaryl ether 79, which arose from a substitution reaction between
pyridine N-oxide 77 and phenol 78 that proceeded in 86% yield.
Next, removal of the N-oxide functionality by means of catalytic
hydrogenation under mild pressure and neutral conditions
afforded diaryl ether 80 in excellent yield. Reduction of the pyridine
to the corresponding piperidine (81) was affected through
the use of catalytic hydrogenation as well, this time under acidic
conditions and elevated pressures relative to the N-oxide reduction. At this juncture, subjection of piperidine 81 to Buchwald¨C
Hartwig conditions in the presence of diol subunit 82 delivered diol 83. A two-step
elimination to deliver enantiopure epoxide 84 set the stage for an
interesting cascade reaction to arrive at delamanid (XI) directly?a
the initial alkylation of the epoxide by imidazole 85 proceeded
under basic conditions with sodium acetate which then underwent
an intramolecular nucleophilic substitution reaction by the liberated
alcohol on the pendant imidazole chloride in the presence
of sodium hydroxide. The reaction sequence proceeded in 73%
yield to provide delamanid (XI) as a free base.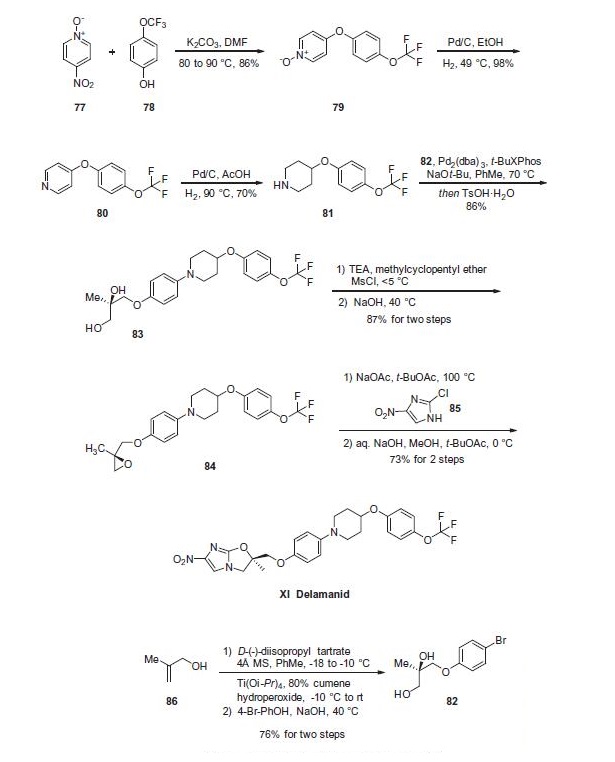
| [Drug interactions]
Potentially hazardous interactions with other drugs
Analgesics: increased risk of ventricular arrhythmias
with methadone
Anti-arrhythmics: increased risk of ventricular
arrhythmias with amiodarone and disopyramide
Antibacterials: possible increased risk of ventricular
arrhythmias with clarithromycin, erythromycin
and moxifloxacin; increased risk of ventricular
arrhythmias with pentamidine; concentration
reduced by rifampicin
Antidepressants: possible increased risk of
ventricular arrhythmias with tricyclics.
Antiepileptics: avoid with carbamazepine.
Antipsychotics: increased risk of ventricular
arrhythmias with droperidol, haloperidol,
phenothiazines that prolong the QT interval and
pimozide.
Antivirals: increased risk of ventricular arrhythmias
with saquinavir
Beta-blockers: increased risk of ventricular
arrhythmias with sotalol.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide and possibly vinblastine,
vincristine, vindesine, vinflunine and vinorelbine
Domperidone: possible increased risk of ventricular
arrhythmias. | [Metabolism]
Delamanid is mainly metabolised in plasma by albumin
and to a lesser extent by CYP3A4. The complete
metabolic profile of delamanid has not yet been
elucidated. The identified metabolites do not show
anti-mycobacterial activity but some contribute to QT
prolongation, mainly DM-6705. |
|
|