| Identification | Back Directory | [Name]
FLUPHENAZINE | [CAS]
69-23-8 | [Synonyms]
s94
S 94
Elinol
sq4918
SQ 4918
dapotum
Sevinol
Pacinol
hiazine
69-23-8
Permitil
Siqualon
Siqualine
Prolixine
Vespazine
Yespazine
FLUFENAZINE
FLUPHENAZINE
Fluorfenazine
ftorphenazine
Fluorphenazine
Fluorophenazine
Triflumethazine
phthorphenazine
moditen(tablorelixir)
Fluphenazine Decaonate
kitasamycin,leucomycin
FLUPHENAZINE USP/EP/BP
Fluphenazine free base
Fluphenazine Decanoate Impurity 2
Fluphenazine Decanoate EP Impurity B
Fluphenazine dihydrochloride solution
4-(3-(-trifluoromethyl-10-phenothiazyl)-propyl)-1-piperazineethanol
4-(3-(2-Trifluoromethyl-10-phenothiazyl)-propyl)-1-piperazineethanol
4-(3-(2-(trifluoromethyl)phenothiazin-10-yl)propyl)-1-piperazineethano
10-(3-(2-Hydroxyethyl)piperazinopropyl)-2-(trifluoromethyl)phenothiazine
1-Piperazineethanol, 4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]propyl]-
1-piperazineethanol,4-(3-(2-(trifluoromethyl)-10h-phenothiazin-10-yl)propyl)
phenothiazine,10-(3-(4-(2-hydroxyethyl)-1-piperazinyl)propyl)-2-(trifluorome
4-(3-(2-(trifluoromethyl)-10h-phenothiazin-10-yl)propyl)-1-piperazineethanol
4-[3-[2-(Trifluoromethyl)-10H-phenothiazin-10-yl]propyl]-1-piperazineethanol
1-(2-hydroxyethyl)-4-(3-(trifluoromethyl-10-phenothiazinyl)propyl)-piperazine
10-(3’-(4"-(beta-hydroxyethyl)-1"-piperazinyl)-propyl)-3-trifluoromethylphenot
1-Piperazineethanol, 4-[3-[2-(trifluoromethyl)-10H-phenothiazin-10-yl]propyl]-
phenothiazine,10-(3-(4-(2-hydroxyethyl)-1-piperazinyl)propyl-2-(trifluoromethy
2-(trifluoromethyl)-10-(3-(1-(beta-hydroxyethyl)-4-piperazinyl)propyl)phenothi
2-(4-(3-[2-(Trifluoromethyl)-10H-phenothiazin-10-yl]propyl)-1-piperazinyl)ethanol
2-(4-{3-[2-(Trifluoromethyl)-10H-phenothiazin-10-yl]propyl}piperazin-1-yl)ethanol
Phenothiazine, 10-(3-(4-(2-hydroxyethyl)-1-piperazinyl)propyl)-2-(trifluoromethyl)-
Fluphenazine HydrochlorideQ: What is
Fluphenazine Hydrochloride Q: What is the CAS Number of
Fluphenazine Hydrochloride Q: What is the storage condition of
Fluphenazine Hydrochloride | [EINECS(EC#)]
200-702-9 | [Molecular Formula]
C22H26F3N3OS | [MDL Number]
MFCD00242759 | [MOL File]
69-23-8.mol | [Molecular Weight]
437.52 |
| Chemical Properties | Back Directory | [Melting point ]
268-274℃ | [Boiling point ]
bp0.5 268-274°; bp0.3 250-252° | [density ]
1.2156 (estimate) | [RTECS ]
TL9730000 | [Fp ]
9℃ | [storage temp. ]
-20°C | [pka]
pKa 7.98±0.03(H2O t=20±0.5) (Uncertain) | [color ]
Dark-brown viscous oil | [Water Solubility ]
31.06mg/L(37 ºC) |
| Hazard Information | Back Directory | [Originator]
Prolixin, Squibb ,US ,1959 | [Uses]
Antipsychotic. | [Uses]
Fluphenazine is an extremely strong antipsychotic drug. A stimulatory effect accompanies
the neuroleptic effect. It is used in psychiatry for treating various forms of schizophrenia
and other mental illnesses. | [Definition]
ChEBI: A member of the class of phenothiazines that is 10H-phenothiazine having a trifluoromethyl subsitituent at the 2-position and a 3-[4-(2-hydroxyethyl)piperazin-1-yl]propyl group at the N-10 position. | [Manufacturing Process]
A suspension of 69.0 grams of 2-trifluoromethylphenothiazine in 1 liter of toluene with 10.9 grams of sodium amide is heated at reflux with high speed stirring for 15 minutes. A solution of 54.1 grams of 1-formyl-4-(3'chloropropyl)-piperazine, [prepared by formylating 1-(3'-hydroxypropyl)piperazine by refluxing in an excess of methyl formate, purifying the 1-formyl4-(3'-hydroxypropyl)-piperazine by vacuum distillation, reacting this compound with an excess of thionyl chloride at reflux and isolating the desired 1-formyl-4(3'-chloropropyl)-piperazine by neutralization with sodium carbonate solution followed by distillation] in 200 ml of toluene is added. The reflux period is continued for 4 hours. The cooled reaction mixture is treated with 200 ml of water. The organic layer is extracted twice with dilute hydrochloric acid. The acid extracts are made basic with ammonia and extracted with benzene. The volatiles are taken off in vacuo at the steam bath to leave a dark brown oil which is 10-[3'-(N-formylpiperazinyl)-propyl]-2trifluoromethylphenothiazine. It can be distilled at 260°C at 10 microns, or used directly without distillation if desired.
A solution of 103.5 grams of 10-[3'-(N-formylpiperazinyl)-propyl]-2trifluoromethylphenothiazine in 400 ml of ethanol and 218 ml of water containing 26 ml of 40% sodium hydroxide solution is heated at reflux for 2 hours. The alcohol is taken off in vacuo on the steam bath. The residue is swirled with benzene and water. The dried benzene layer is evaporated in vacuo. The residue is vacuum distilled to give a viscous, yellow oil, 10(3'piperazinylpropyl)-2-trifluoromethylphenothiazine, distilling at 210° to235°C at 0.5 to 0.6 mm.
A suspension of 14.0 grams of 10-(3'-piperazinylpropyl)-2trifluoromethylphenothiazine, 6.4 grams of β-bromoethyl acetate and 2.6 grams of potassium carbonate in 100 ml of toluene is stirred at reflux for 16 hours. Water (50 ml) is added to the cooled mixture. The organic layer is extracted into dilute hydrochloric acid. After neutralizing the extracts and taking the separated base up in benzene, a viscous, yellow residue is obtained by evaporating the organic solvent in vacuo. This oil is chromatographed on alumina. The purified fraction of 7.7 grams of 10-[3'-(Nacetoxyethylpiperazinyl)-propyl] -2-trifluoromethylphenothiazine is taken up in ethyl acetate and mixed with 25 ml of alcoholic hydrogen chloride. Concentration in vacuo separates white crystals of the dihydrochloride salt, MP 225° to 227°C.
A solution of 1.0 gram of 10-[3'-(N-acetoxyethylpiperazinyl)-propyl]-2trifluoromethylphenothiazine in 25 ml of 1 N hydrochloric acid is heated at reflux briefly. Neutralization with dilute sodium carbonate solution and extraction with benzene gives the oily base, 10-[3'-(N-βhydroxyethylpiperazinyl)-propyl]-2-trifluoromethylphenothiazine. The base is reacted with an excess of an alcoholic hydrogen chloride solution. Trituration with ether separates crystals of the dihydrochloride salt, MP 224° to 226°C, (from US Patent 3,058,979). | [Therapeutic Function]
Tranquilizer | [Safety Profile]
Poison by ingestion,intraperitoneal, and intravenous routes. Moderately toxicby subcutaneous route. Experimental reproductive effects.When heated to decomposition it emits very toxic fumesof Fí, NOx, and SOx. | [Synthesis]
Fluphenazine, 4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]propyl]-1-
piperazineethanol (6.1.8), is synthesized by any of the methods described above [21¨C27].
Alkylation of 2-trifluoromethylphenothiazine using 4-formyl-1-piperazineylpropylchlo�ride in the presence of sodium amide synthesizes 2-trifluoromethyl-10-[3-(4-formyl-
1-piperazinyl)propyl]phenothizine (6.1.6). Further alkaline hydrolysis removes the
N-formyl group, giving 2-trifluoromethyl-10-[3-(1-piperazinyl)propyl]phenothiazine
(6.1.7). This is alkylated by 2-bromethanol-1 acetate, which upon further acidic hydroly�sis removes the protecting acetyl group, yielding fluphenazine (6.1.8) [27,28].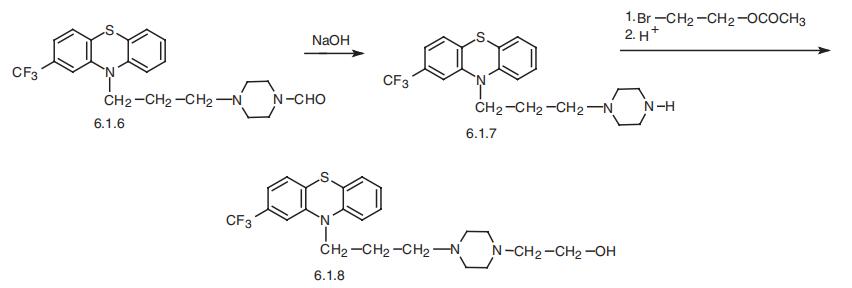
|
|
| Company Name: |
BOC Sciences
|
| Tel: |
16314854226 |
| Website: |
www.bocsci.com |
|