| Identification | Back Directory | [Name]
(S)-6-(3-CHLORO-2-FLUOROBENZYL)-1-(1-HYDROXY-3-METHYLBUTAN-2-YL)-7-METHOXY-4-OXO-1,4-DIHYDROQUINOLINE-3-CARBOXYLIC ACID | [CAS]
697761-98-1 | [Synonyms]
EVG
CS-789
CS-456
D06677
Vitekta
GS 9137
JTK 303
Elvitegravi
Elvitegravir
brand name: Stribild
Elvitegravir (GS-9137)
GS9137;GS-9137;GS 9137
ELVITEGRAVIR CAS 697761-98-1
Elvitegravir(GS-9137,JTK-303)
Elvitegravir,EVG,GS-9137,JTK-303
(S)-6-(3-CHLORO-2-FLUOROBENZYL)-1-(1-HYDROXY-3-METHYLBUTAN-2-YL)-7-METHOXY-4-OXO
1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid
GS-9137; GS9137; GS 9137; JTK 303; JTK-303; JTK303; EVG; ELVITEGRAVIR; BRAND NAME: STRIBILD
(S)-6-(3-Chloro-2-fluorobenzyl)-1-(1-hydroxy-3-methylBUTAN-2-YL)-7-methoxy-4-oxo-1,4-dihydroqu
(S)-6-(3-CHLORO-2-FLUOROBENZYL)-1-(1-HYDROXY-3-METHYLBUTAN-2-YL)-4-OXO-1,4-DIHYDROQUINOLINE-3-CARBOXYLIC ACID
6-[(3-chloro-2-fluorophenyl)methyl]-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxoquinoline-3-carboxylic acid
(S)-6-(3-CHLORO-2-FLUOROBENZYL)-1-(1-HYDROXY-3-METHYLBUTAN-2-YL)-7-METHOXY-4-OXO-1,4-DIHYDROQUINOLINE-3-CARBOXYLIC ACID
6-(3-Chloro-2-fluorobenzyl)-1-[1(S)-(hydroxymethyl)-2-methylpropyl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
(S)-6-(3-CHLORO-2-FLUOROBENZYL)-1-(1-HYDROXY-2,3-DIMETHYLBUTAN-2-YL)-7-METHOXY-4-OXO-1,4-DIHYDROQUINOLINE-3-CARBOXYLIC ACID
(S)-6-(3-CHLORO-2-FLUOROBENZYL)-1-(1-HYDROXY-3-METHYLBUTAN-2-YL)-7-METHOXY-4-OXO-1,4-DIHYDROQUINOLINE-3-CARBOXYLIC ACID USP/EP/BP
6-[(3-chloro-2-fluorophenyl)Methyl]-1-[(2S)-1-hydroxy-2,3-diMethylbutan-2-yl]-7-Methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
6-[(3-Chloro-2-fluorophenyl)Methyl]-1,4-dihydro-1-[(1S)-1-(hydroxyMethyl)-2-Methylpropyl]-7-Methoxy-4-oxo-3-quinolinecarboxylic Acid
3-Quinolinecarboxylic acid, 6-[(3-chloro-2-fluorophenyl)methyl]-1,4-dihydro-1-[(1S)-1-(hydroxymethyl)-2-methylpropyl]-7-methoxy-4-oxo-
ElvitegravirQ: What is
Elvitegravir Q: What is the CAS Number of
Elvitegravir Q: What is the storage condition of
Elvitegravir Q: What are the applications of
Elvitegravir
6-(3-Chloro-2-fluorobenzyl)-1-[1(S)-(hydroxymethyl)-2-methylpropyl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid Elvitegravir (GS-9137, JTK-303) | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C23H23ClFNO5 | [MDL Number]
MFCD11846134 | [MOL File]
697761-98-1.mol | [Molecular Weight]
447.888 |
| Chemical Properties | Back Directory | [Melting point ]
93-96°C | [Boiling point ]
623.6±55.0 °C(Predicted) | [density ]
1.357±0.06 g/cm3(Predicted) | [storage temp. ]
Refrigerator | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
0.44±0.20(Predicted) | [color ]
Off-White to Pale Yellow | [InChIKey]
JUZYLCPPVHEVSV-LJQANCHMSA-N | [SMILES]
N1([C@H](CO)C(C)C)C2=C(C=C(CC3=CC=CC(Cl)=C3F)C(OC)=C2)C(=O)C(C(O)=O)=C1 | [CAS DataBase Reference]
697761-98-1 |
| Hazard Information | Back Directory | [Description]
In November 2013, the European Medicines Agency (EMA) approved elvitegravir (also known as GS 9137 and JTK 303) as a single agent to be used as part of an antiviral regimen that includes a ritonavir-boosted protease inhibitor for the treatment of HIV-1 in adults without mutations indicative of elvitegravir resistance. Elvitegravir is the second of three marketed HIV integrase strand transfer inhibitors (INSTIs) including raltegravir and dolutegravir (this volume of ARMC). Elvitegravir was discovered by modification of a literature naphthyridine HIV integrase inhibitor in which the naphthyridine core served as a bioisostere for the diketo acid moiety in an original series. Serendipitously, a 4-quinolone-3-carboxylic acid precursor en route to the desired bioisosteric glyoxylic acid demonstrated modest integrase inhibition (IC50=1600 nM). Further derivatization led to elvitegravir with enhanced inhibition of integrase strand transfer (IC50=7.2 nM) and significant antiviral activity (EC50=0.9 nM). Elvitegravir was prepared in seven synthetic steps from 2,4-difluoro-5-iodobenzoic acid. The corresponding acid chloride was coupled to ethyl 3-(dimethylamino) acrylate and further substituted with S-valinol. Base promoted cyclization afforded the quinolone which was protected as silyl ether. Negishi coupling installed the 2-fluoro-3-chlorobenzyl moiety. Subsequent hydrolysis and methoxylation afforded elvitegravir. | [Chemical Properties]
Off-White to Pale Yellow Solid | [Originator]
Torii Pharmaceuticals
(subsidiary of Japan Tobacco) (Japan) | [Uses]
A novel inhibitor of human immunodeficiency virus type 1 integrase. | [Uses]
Elvitegravir (EVG, JTK-303/GS-9137) is an HIV integrase inhibitor for HIV-1 IIIB, HIV-2 EHO and HIV-2 ROD with IC50 of 0.7 nM, 2.8 nM and 1.4 nM, respectively. | [Uses]
Elvitegravir is a quinolone antibiotic that inhibits the integrase of HIV-1 (IC50 = 7.2 nM). It blocks the integration of HIV-1 cDNA through the inhibition of DNA strand transfer. Elvitegravir is used in combination with a pharmacoenhancer and nucleoside/nucleotide reverse transcriptase inhibitors to block HIV-1 replication in vivo.[Cayman Chemical] | [Definition]
ChEBI: A quinolinemonocarboxylic acid that is 7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid substited at position 1 by a 1-hydroxy-3-methylbutan-2-yl group and at position 6 by a 3-chloro-2-fluorobenzyl group (the S-enantiomer). It is us
d in combination therapy for the treatment of HIV-1 infection. | [Brand name]
Vitekta? (Europe), Stribild?
(as combo in the United States
and Japan) | [Synthesis]
Commercial 2,4-dimethoxy-5-bromo benzoic acid (61) was
reacted with 0.5 equiv of butylethylmagnesium to generate the
dimagnesium salt in THF, which was then lithiated at �20 ?? to
give the aryl lithium species. The aryl lithium species was then
reacted with the 2-fluoro-3-chloro benzaldehyde (62) to give alcohol 63. Treatment with triethylsilane in TFA resulted in
removal of the hydroxyl functionality to provide benzoic acid 64.
This acid was then reacted with carbonyldiimidazole and subsequently
magnesium malonate 65 to give ketoester 66 after
workup. Next, condensation with DMF¨CDMA converted ketoester
66 to the vinylogous amide 67, and this material was immediately
subjected to an addition¨Celimination reaction involving (S)-valinol
(68) in toluene at ambient temperature to provide intermediate 69.
Warming the resulting intermediate 69 in the presence of N,Obistrimethylsilyl
acetamide (BSA) and potassium chloride in DMF
furnished the ring-closed quinolone 70. The ester 70 was saponified
with potassium hydroxide in aqueous isopropanol and then
acidified and crystallized with the use of seed crystals. Upon
cooling, the crystalline product elvitegravir (IX) was collected by
filtration.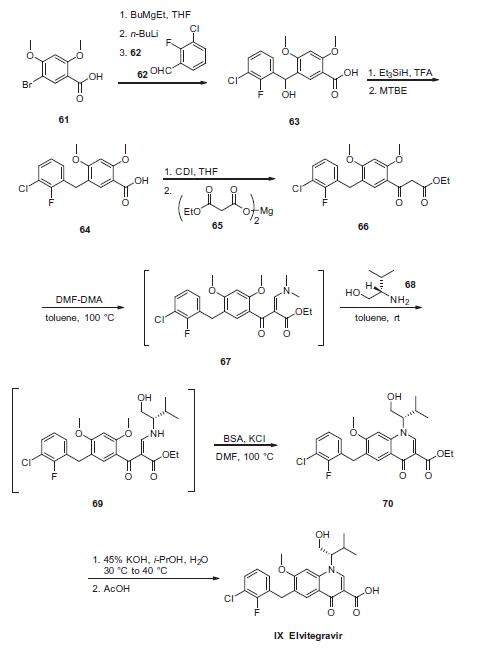
| [target]
HIV-I integrase | [storage]
Store at -20°C |
|
|