| Identification | Back Directory | [Name]
Naltrexone methylbromide | [CAS]
73232-52-7 | [Synonyms]
MNTX
Moa-728
Relistor
73232-52-7
Unii-rfo6il3D3m
Naltrexone methobromide
Naltrexone methylbromide
Methylnaltrexone bromide
Methyhaaltrexone broMide
N-Methyl Naltrexone BroMide
Methylnaltrexone iodide salt
Quaternary ammonium naltrexone
N-Methyl Naltrexone Bromide (1.0mg/ml in DMSO)
N-CyclopropylMethyl-noroxyMorphone MethobroMide
Methylnaltrexone Peak Identification Mixture Cll
17-(CyclopropylMethyl)-4,5-epoxy-3,14-dihydroxy-17-Methyl-6-oxoMorphinaniuM broMide
17-(Cyclopropylmethyl)-4,5A-Epoxy-3,14-Dihydroxy-17-Methyl-6-Oxomorphinanium Bromide
(5α)-17-(CyclopropylMethyl)-4,5-epoxy-3,14-dihydroxy-17-Methyl-6-oxoMorphinaniuM BroMide
(5alpha)-17-(Cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-17-methyl-6-oxomorphinanium bromide
Morphinanium, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-17-methyl-6-oxo-, bromide, (5alpha)-
(4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-dihydroxy-3-methyl-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-3-ium-7-one,bromide | [Molecular Formula]
C21H26BrNO4 | [MDL Number]
MFCD06407831 | [MOL File]
73232-52-7.mol | [Molecular Weight]
436.339 |
| Chemical Properties | Back Directory | [Melting point ]
237-239°C | [storage temp. ]
Hygroscopic, -20?C Freezer, Under Inert Atmosphere | [solubility ]
H2O: ≥5mg/mL | [form ]
powder | [color ]
white to beige | [Stability:]
Hygroscopic |
| Hazard Information | Back Directory | [Description]
The widespread efficacy of opioids in treating patients with moderate to
severe acute and chronic pain is often accompanied by untoward side
effects. In particular, opioid-induced bowel dysfunction is one of the
more common and debilitating consequences afflicting up to 50% of
patients. To counteract the peripherally-mediated adverse effects, opioid
antagonists such as naloxone, naltrexone, and nalmephene are sometimes prescribed. The latest market entry exploits a strategic
modification of naltrexone to lower its lipid solubility and increase its
polarity: quaternization of the amine of naltrexone by methylation
(methyl bromide) prevents crossing of the blood–brain barrier thereby
creating an effective peripheral antagonist. Despite a loss of potency
upon methylation, methylnaltrexone antagonizes opioid binding at
m-opioid receptors with an IC50 of 70 nM. Its affinity for k-opioid receptors
is approximately eightfold less (IC50= 575 nM) with no significant binding
to d-opioid, orphanin, or non-opioid receptors. Methylnaltrexone bromide
has been approved for the treatment of opioid-induced constipation in
patients with advanced illness receiving palliative care.Regarding metabolism, methylnaltrexone bromide is eliminated primarily as intact drug (85% based on administered radioactivity) by slightly more renal than hepatic clearance.
The most common adverse events were abdominal pain and flatulence followed by nausea, dizziness, and diarrhea. | [Chemical Properties]
Pale Pink Solid | [Originator]
University of Chicago (United States) | [Uses]
A metabolite of Naltrexone (N285750). Methylnaltrexone (MNTX), a selective μ-opioid receptor antagonist, functions as a peripherally acting receptor antagonist in tissues of the gastrointestinal tract. | [Brand name]
Relistor | [General Description]
Methylnaltrexone does not cross blood brain barrier and does not affect the opioid effects in the brain, such as analgesia. It is used to treat opioid-induced constipation (OIC). | [Biochem/physiol Actions]
Methylnaltrexone bromide is a narcotic antagonist. It is a peripheral mu-opiod receptor antagonist that cannot cross the blood-brain barrier. It reverses many opioid side-effects without interfering with pain relief. | [Clinical Use]
N-Methylnaltrexone
is the polar derivative
of naltrexone, which does not reach the central
nervous system but can block intestinal opioid
receptors. The compound is under development
to counteract opioid induced bowel dysfunction
such as constipation and megacolon . | [Synthesis]
The
synthesis of methylnaltrexone bromide proceeds in a
straightforward manner via the alkylation of naltrexone 90
in the following scheme. Naltrexone 90 was reacted with methyl
bromide in 1-methyl-2-pyrrolidinone at 60 ??C. The resulting
crude product was treated with sodium methoxide in methanol/
water at 55 ??C to remove any undesired phenolic (Oalkylated)
side-products. The resulting crude sodium salt was
treated with hydrobromic acid in methanol/water and upon
crystallization gave methylnaltrexone bromide (XII) in 35%
overall yield.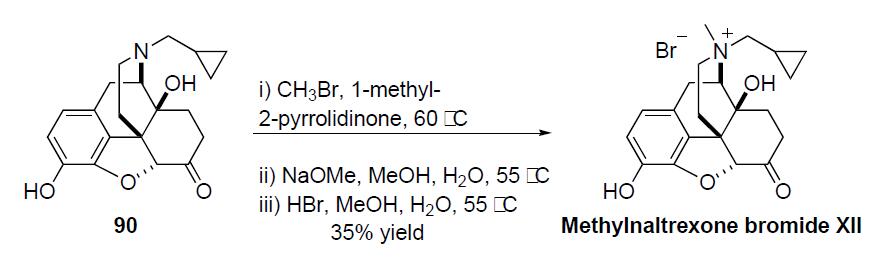
| [storage]
Store at -20°C |
|
| Company Name: |
Rusan Pharma Ltd
|
| Tel: |
+91-2228687035 +91-9690022465 |
| Website: |
www.rusanpharma.com |
|