| Identification | Back Directory | [Name]
Lobeglitazone Sulfate | [CAS]
763108-62-9 | [Synonyms]
CKD501
CKD-501
CKD 501
Chong Kun Dang
Lobeglitazone Sulfate
Lobeglitazone Sulfate. trade name Duvie
Lobeglitazone Sulfate. trade name Duvie, Chong Kun Dang | [Molecular Formula]
C24H26N4O9S2 | [MDL Number]
MFCD28502044 | [MOL File]
763108-62-9.mol | [Molecular Weight]
578.61 |
| Hazard Information | Back Directory | [Description]
Lobeglitazone sulfate, an oral peroxisome proliferator-activated
receptor (PPARa/c) dual agonist with IC50 = 20 and 18 nM respectively,
was developed by Chong Kun Dang Pharmaceutical in Korea
for the treatment of diabetes. This drug is differentiated from
two other PPAR agonists available—pioglitazone and rosiglitazone
—which lack PPARa activity. The most likely processscale
preparation of lobeglitazone sulfate follows the route
described in a process communication from Chong Kun Dang
Pharmaceutical. | [Uses]
Lobeglitazone sulfate is a new type of thiazolidinedione. Lobeglitazone sulfate is the orally active agonist for PPAR with EC50 of 137.4 nM and 546.3 nM for PPARγ and PPARα. Lobeglitazone sulfate is the inhibitor for ERK/JNK/Smad/NF-κB signaling pathway. Lobeglitazone sulfate exhibits anti-inflammatory, anti-diabetic, anti-fibrotic and anti-atherosclerotic properties[1][2][3][4][5][6]. | [Synthesis]
Commercially available 4,6-dichloropyrimidine (152) was treated
with a stoichiometric equivalent of p-methoxyphenol (153) in
the presence of KF in warm DMF . Upon completion of
this reaction, 2-methylaminoethanol was added to the mixture to
provide pyrimidine 154 in high yield. Next, alcohol 154 underwent
a substitution reaction with p-fluorobenzaldehyde (155)
under basic conditions to provide alkoxy benzaldehyde 156 which
was converted to the benzylidene thiazolidindione 158 upon subjection
to Knoevenagel conditions with 2,4-thiazolidinedione (157)
in 90% yield. Finally, reduction of olefin 158 was facilitated by
treatment with the Hantzsch ester (159) in the presence of silica
gel followed by treatment with methanolic sulfuric acid (96%) at
low temperature to ultimately furnish lobeglitazone sulfate in
90% yield.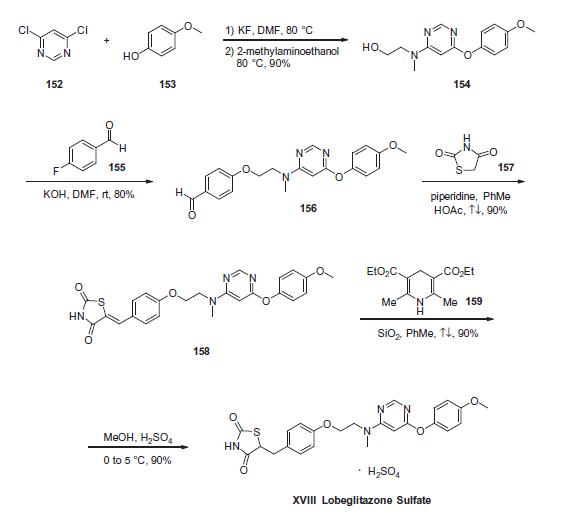
| [in vivo]
Lobeglitazone sulfate (1-10 mg/kg, po for 8 weeks) exhibits anti-atherosclerotic property in ApoE?/? mouse models[4].
| Animal Model: | ApoE?/? mouse aortic atherosclerosis models[4] | | Dosage: | 1-10 mg/kg | | Administration: | po for 8 weeks | | Result: | Reduced aortic arch plaques. |
| [IC 50]
PPARγ: 137.4 nM (EC50); PPARα: 546.3 nM (EC50) | [References]
[1] Bae J, et al. Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus. Diabetes Metab J. 2021 May;45(3):326-336.
[2] Jeong D, et al., Lobeglitazone Exerts Anti-Inflammatory Effect in Lipopolysaccharide-Induced Bone-Marrow Derived Macrophages. Biomedicines. 2021 Oct 10;9(10):1432. DOI:10.3390/biomedicines9101432
[3] Nuwormegbe S, et al. Lobeglitazone attenuates fibrosis in corneal fibroblasts by interrupting TGF-beta-mediated Smad signaling. Graefes Arch Clin Exp Ophthalmol. 2022 Jan;260(1):149-162. DOI:10.1007/s00417-021-05370-2
[4] Lim S, et al., Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015 Nov;243(1):107-19. DOI:10.1016/j.atherosclerosis.2015.08.037 |
|
|