| Identification | Back Directory | [Name]
HALOPROGIN (200 MG) | [CAS]
777-11-7 | [Synonyms]
m1028
polik
mycilan
halotex
mycanden
Fungaclor
NSC-100071
haloprogin
HALOPROGIN (200 MG)
HALOPROGIN (200 MG) USP/EP/BP
2,4,5-trichlorophenyliodopropargylether
3-iodo-2-propynyl2,4,5-trichlorophenylether
ether,3-iodo-2-propynyl2,4,5-trichlorophenyl
1,2,4-trichloro-5-(3-iodoprop-2-ynoxy)benzene
2,4,5-trichlorophenyl-gamma-iodopropargylether
1,2,.4-Trichloro-5-[(3-iodo-2-propynyl)oxy]benzene
1,2,4-trichloro-5-((3-iodoprop-2-yn-1-yl)oxy)benzene | [EINECS(EC#)]
212-286-6 | [Molecular Formula]
C9H4Cl3IO | [MDL Number]
MFCD00056358 | [MOL File]
777-11-7.mol | [Molecular Weight]
361.39 |
| Hazard Information | Back Directory | [Originator]
Halotex,Westwood,US,1972 | [Uses]
Antibacterial. | [Uses]
Haloprogin is a topical antifungal agent used in the treatment of dermatophytic and monilial infections. | [Definition]
ChEBI: Haloprogin is an aromatic ether. | [Indications]
Haloprogin is used as an external drug for moderate dermatophyte infections (shingles),
and it is effective for superficial candida infections. Synonyms of this drug are halotex,
mycilan, micanden, and others. | [Manufacturing Process]
4.7 grams of 2,4,5-trichlorophenyl propargyl ether (MP 64° to 65°C) are
added to an aqueous solution of cupro-ammonium complex salt which has been prepared by warming a mixture of 4.0 grams of cuprous chloride, 11.0
grams of ammonium carbonate and 20 cc of water to 50°C. The resulting
admixture is shaken vigorously. The cuprous acetylide deposited is filtered,
washed with water and suspended in 100 cc of water, and the suspension is
mixed under agitation with a solution of 5.0 grams of iodine and 5.0 grams of
potassium iodide in 15 cc of water. The mixture is stirred for a period of 1
hour. The precipitate is filtered, washed with water and extracted with ether.
After the drying of the ethereal extract, the solvent is distilled off.
Recrystallization of the residue from n-hexane gives about 5.6 grams of 2,4,5-
trichlorophenyi iodopropargyl ether, MP 114° to 115°C. | [Brand name]
Halotex (Westwood-Squibb). | [Therapeutic Function]
Antibacterial | [General Description]
3-Iodo-2-propynyl-2,4,5-trichlorophenyl ether (Halotex)crystallizes as white to pale yellow forms that are sparinglysoluble in water and very soluble in ethanol. It is an etherealderivative of a phenol. Haloprogin is used as a 1% cream forthe treatment of superficial tinea infections.
Formulations of haloprogin should be protected fromlight because the compound is photosensitive. Haloprogin isavailable as a solution and a cream, both in a 1% concentration.Haloprogin is probably not the first topical agent thatshould be recommended. Although the cure rates for topicalfungal infections are relatively high, they come at a highprice. The lesion typically worsens before it improves.Inflammation and painful irritation are common. | [Synthesis]
Haloprogin, 3-iodo-2-propinyl-2,4,5-trichlorophenyl ether (35.4.11), is syn�thesized by an iodide substitution using a mixture of iodine and potassium iodide and a
cupric derivative of 2,4,5-trichlorophenylpropargyl ether (35.4.10), which is synthesized
by a standard method from propargyl bromide and 2,4,5-trichlorophenol in the presence of
sodium hydroxide.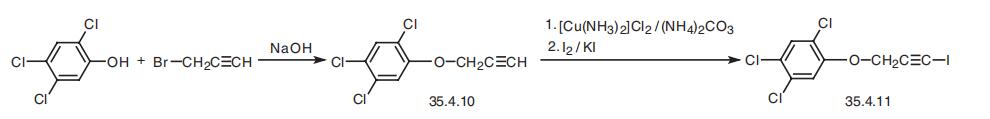
|
|
| Company Name: |
Alfa Chemistry
|
| Tel: |
+1 (201) 478-8534 |
| Website: |
www.alfa-chemistry.com |
|