| Identification | Back Directory | [Name]
Apatinib | [CAS]
811803-05-1 | [Synonyms]
Aptinib
Apatinib
Mesylate APA
Apatinib Mesylate
Apatinib (YN968D1)
N-(4-(1-cyanocyclopentyl)phenyl)-2-(pyridin-4-ylMethylaMino)nicotinaMide
N-[4-(1-Cyanocyclopentyl)phenyl]-2-[(4-pyridinylmethyl)amino]-3-pyridinecarboxamide Apatinib | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C24H23N5O | [MDL Number]
MFCD21648511 | [MOL File]
811803-05-1.mol | [Molecular Weight]
397.472 |
| Chemical Properties | Back Directory | [Boiling point ]
578.2±50.0 °C(Predicted) | [density ]
1.27 | [storage temp. ]
Store at -20°C | [solubility ]
>49.4mg/ml in DMSO | [form ]
Powder | [pka]
11.93±0.70(Predicted) |
| Hazard Information | Back Directory | [Uses]
Apatinib (YN968D1) is a small-molecule selective multitargeted tyrosine kinase inhibitor with an IC50 of 2.43 nM for the inhibition of VEGFR2. | [Description]
Apatinib is a tyrosine kinase inhibitor that potently suppresses the kinase activity of vascular endothelial growth factor 2 (VEGFR2; IC50 = 1 nM). It is less effective against c-kit (IC50 = 429 nM), Ret (IC50 = 13 nM), and c-src (IC50 = 53 nM) and does not inhibit EGFR, Her-2, or FGFR1 (IC50s = >10 μM). Apatinib has been shown to inhibit the proliferation, migration, and tube formation of human umbilical vein endothelial cells stimulated by fetal bovine serum and, either alone, or in combination with chemotherapeutic agents, prevented the growth of several established human tumor xenograft models. | [Description]
Apatinib mesylate, discovered by Advenchen Laboratories (United
States of America, USA) and co-developed by Jiangsu Hengrui
Medicine Co. Ltd (China), was approved by the Chinese Food and
Drug Administration (CFDA) in October 2014 for the treatment of
metastatic gastric carcinoma. Apatinib mesylate is an oral tyrosine
kinase inhibitor that selectively inhibits the vascular
endothelial growth factor receptor 2 (VEGFR2), which prevents new blood vessel formation selectively in tumor tissue. Apatinib
has shown inhibition of the VEGF signaling pathway with an IC50
value of 1 nM for VEGFR-2 in in vitro enzyme experiments. A
multicenter phase II study of apatinib is underway with patients
in non-triple-negative metastatic breast cancer trials. Non-clinical
studies concluded that apatinib may reverse the ATP-binding
cassette subfamily B member 1 and subfamily G member 2
(ABCB1- and ABCG2, respectively)-mediated multidrug resistance
which allows cancer cells to circumvent certain conventional antineoplastic
drugs, suggesting that apatinib could be effective as a
combination therapy. | [Synthesis]
The synthesis started
with commercially available 1-phenyl cyclopentane carbonitrile
(8), which was nitrated to provide nitrobenzene 9. Subsequent
reduction of 9 gave aniline 10, which was coupled with 2-chloronicotinoyl
chloride (11) to afford aryl amide 12. Subjection of the
2-pyridyl chloride within 12 to pyridin-4-ylmethanamine (13) in
hot pentanol gave 14. The preparation of apatinib 14 from starting
material 8 were reported on gram or milligram reaction scale with
no yield. 170 g of 14 was mixed with methylsulfonic acid in 95%
isopropanol¨CH2O solution to give 161.5 g of apatinib mesylate (II)
in 77% yield.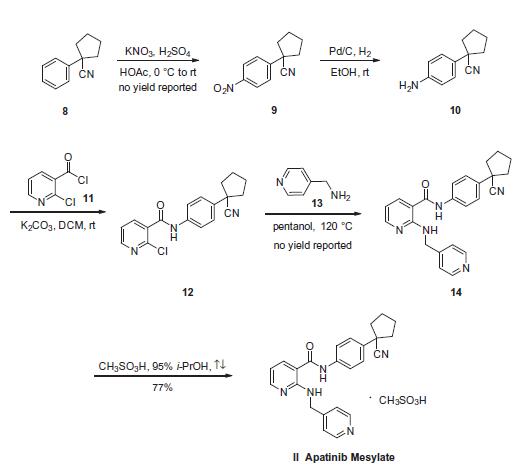
| [target]
VEGFR2 |
| Questions And Answer | Back Directory | [Treatment of advanced gastric cancer]
Apatinib is the first small-molecule anti-angiogenic targeting drug that has been proven safe and effective in the treatment of advanced gastric cancer. It is also the single-drug of the best efficacy after the failure of the standardization treatment on advanced gastric cancer. Moreover, apatinib is also the only oral-administrated drug among the drugs targeted to the gastric cancer, which will greatly improve the cure rate of patients. In June 2014, the clinical study of the drug by the American Society of Clinical Oncology (ASCO) had been selected for the General Assembly report, which was the first time that China's innovative drug research had received general assembly report in the world's top academic conference, also the first time that selected as the excellent research in this meeting.
On December 13, 2014, Jiangsu Hengrui Pharmaceutical Co., Ltd. announced that the State Food and Drug Administration had approved the marketing of a new drug for the treatment of advanced gastric cancer, “Aitai” (apatinib) in China. “Aitan”, Apatinib Mesylate, is the first orally administrated small-molecule anti-angiogenic targeting drug that has been proven to be safe and effective after the failure of the standard chemotherapy on the advanced gastric cancer, being able to significantly prolong the overall survival of patients with advanced gastric cancer. It has brought new hope for patients with advanced gastric cancer.
Information regarding the pharmacological effects, indications, market prospects and side effects of the drug for advanced gastric cancer treatment, apotinib is edited by Xiao Nan from Chemicalbook. (2015-09-23) | [Pharmacological effects]
Apatinib is a small molecule targeting drug for anti-VEGFR-2 that targets patients with advanced gastric cancer who have failed in the second-line chemotherapy. It can block the downstream signaling transduction by highly selective competition for ATP-binding sites in receptor-2, inhibit the production of the tyrosine kinase production, thus inhibiting the tumor tissue neovascularization, and ultimately achieving the purpose of treatment of cancer.
The clinical trial was jointly led by Professor Shukui Qin and Professor Jin Li from the PLA Nanjing Bayi Hospital. This has obtained the strong support from 38 hospitals around the country. There have been a total of 273 patients who have joined into the group. They have been randomly distributed into oral apatinib group or placebo group at a ratio of 2:1. After a year and a half of the study time, apapatinib was found to significantly prolong the overall survival (OS) for 55 days (195 days vs 1.4 days, P <0.016) compared with placebo. | [Apatinib Mesylate]
Apatinib Mesylate is a small molecule tyrosine kinase inhibitor developed by Jiangsu Hengrui Pharmaceutical Co., Ltd. Through selectively inhibiting the tyrosine kinase activity of the vascular endothelial growth factor receptor 2 (VEGFR-2), it could inhibit the tumor angiogenesis and tumor growth. It is clinically used in the treatment of the "advanced gastric cancer that receives second-line chemotherapy failure". The dosage is used as "850 mg, once a day." The formulation type is tablet with the specification including 250 mg, 375 mg and 425 mg.

Figure 1: apatinib mesylate tablets of 250mg | [Market expectation]
Gastric cancer is a kind of cancer of high incidence in China, and has high mortality rate. According to the statistics, there were 95,000 new cases around the world in 2013, of which China accounted for 47 percent. Its incidence ranks the second in malignant tumors in China, with about 325,300 deaths per year. China ranks third in malignant tumor mortality. As the early symptoms of cancer is not obvious and failure of the popularization of the endoscopic routine examination, about 70% of patients were diagnosed with advanced gastric cancer at the time of treatment. The existing treatment is effective with poor prognosis. The five-year survival rate of is not more than 20%. In recent decades, although we have spent a lot of manpower and material resources, but there has been not any major breakthrough regarding the development of new drugs after the failure of the standardization of advanced gastric cancer. This leads that this group hasn’t obtained effective treatment, brining great burden to patients, society and countries. Apatinib is a new small molecule targeted drug through suppressing tumor angiogenesis to suppress tumor. It can significantly prolong the survival of patients while significantly reducing patient costs as well. | [Side effect]
In terms of safety, until November 2013, all clinical trials of apotinib safety data include 36 cases of healthy people having taken once and 1230 solid tumor cases with administration for at least once. In the longest medication case, the whole medication process has continued for 2 years. During the I, II, and III trials, there were 229 subjects (6 trials in stage I, 47 in stage II trials and 176 in stage III trials) receiving 850 mg qd dose. The observed adverse effects were similar to those of other anti-angiogenic drugs. Common adverse reactions (≥5%) include hematologic toxicity (leukopenia, neutropenia, thrombocytopenia, hemoglobin reduction) and proteinuria, hypertension, hand-foot syndrome, gastrointestinal reactions (diarrhea, abdominal pain, nausea, vomiting , Loss of appetite), fatigue, back pain, hoarse voice. Major laboratory abnormalities include abnormal liver enzymes (increased transaminase, elevated bilirubin, increased alkaline phosphatase, elevated glutamyl transpeptidase and elevated lactate dehydrogenase), electrolyte abnormality (low Potassium, low calcium, low sodium). Common serious adverse reactions include upper gastrointestinal bleeding. Most of the treatment-related adverse events are grade 1-2 and can be treated by temporarily discontinuing apapatinib mesylate, reducing the dose or applying symptomatic treatment. There are currently no unexpected toxicities, similar to the adverse reactions reported in Phase III. |
|