| Identification | Back Directory | [Name]
1-ethyl-1H-pyrazol-4-amine | [CAS]
876343-24-7 | [Synonyms]
1-ethylpyrazol-4-amine
4-Amino-1-ethylpyrazole
1-ethyl-1H-pyrazol-4-amine
4-Amino-1-ethyl-1H-pyrazole
1H-Pyrazol-4-amine, 1-ethyl-
1-ethyl-1H-pyrazol-4-amine hydrochloride
1-ethyl-1H-pyrazol-4-amine(SALTDATA: HCl) | [Molecular Formula]
C5H9N3 | [MDL Number]
MFCD05861667 | [MOL File]
876343-24-7.mol | [Molecular Weight]
111.145 |
| Chemical Properties | Back Directory | [Boiling point ]
238.6±13.0 °C(Predicted) | [density ]
1.16±0.1 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [pka]
3.72±0.10(Predicted) | [CAS DataBase Reference]
876343-24-7 |
| Hazard Information | Back Directory | [Uses]
1-ethyl-1H-pyrazol-4-amine can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | [Synthesis]
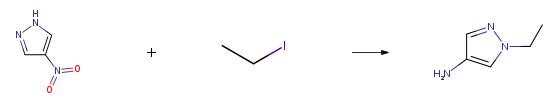
General Procedure for the Synthesis of 4-Amino-l-N-alkylated-pyrazolesA solution of 4-nitropyrazole (300mg, 2.65mmol), potassium carbonate (2eq) and the alkylating reagent (l .leq) in acetonitrile (lOmL) was heated at 60??C for 18h. After cooling to rt the mixture was diluted with EtOAc and washed with water. The organic phase was collected, dried (MgS04) and concentrated in vacuo. The crude residue was dissolved in methanol (lOmL), palladium on carbon (50mg) was added and the reaction was stirred under a balloon of hydrogen for 18h. The resulting mixture was filtered through Celite and the filtrate concentrated in vacuo to give the desired product. Procedure B:Example 11: 2-((6-( 1 -Ethyl- 1 H-pyrazo 1-4-ylamino)- 1 H-pyrazo lo [3 ,4-d]pyrimidin- 1 -yl) methyl)benzonitrileThe following compound was made according to the procedure in Example 1, using 1-ethyl- lH-pyrazo 1-4-amine. 1 -ethyl- lH-pyrazol-4-amine was prepared by Procedure A using ethyl iodide as alkylating agent: 1H NMR (d6-DMSO) |? 9.94 (s, 1H), 8.94 (s, 1H), 8.10 (s, 2H), 7.91 (dd, 1H), 7.66 (td, 1H), 7.49-7.53 (m, 2H), 7.35-7.37 (m, 1H), 5.76 (s, 2H), 4.11 (q, 2H), 1.35 (t, 3H); LC-MS method B, (ES+) 345.1, RT = 8.58min. |
|
|