| Identification | Back Directory | [Name]
1-[3-[3-(4-Chlorophenyl)propoxy]propyl]-piperidinehydrochloride | [CAS]
903576-44-3 | [Synonyms]
BF 2649
CS-2751
CS-1548
Ciproxidine
Pitolisant HCl
Ciproxidine BF2649
Pitolisant HCI salt
BF 2649 hydrochloride
Pitolisant Hcl(BF2.649
Pitolisant (Ciproxidine
Pitolisant (hydrochloride)
PITOLISANT;CIPROXIDINE BF2649
CIPROXIDINE;BF 2649;BF2649;BF-2649
Pitolisant hydrochloride (Ciproxidine
PITOLISANT HYDROCHLORIDE;BF 2649;BF2649;BF-2649
1-[3-[3-(4-Chlorophenyl)propoxy]propyl]piperidine HCl
1-[3-[3-(4-Chlorophenyl)propoxy]propyl]-piperidinehydrochloride
1-[3-[3-(4-Chlorophenyl)propoxy]propyl]piperidine monohydrochloride
Piperidine, 1-[3-[3-(4-chlorophenyl)propoxy]propyl]-, hydrochloride | [Molecular Formula]
C17H26ClNO.HCl | [MDL Number]
MFCD09970745 | [MOL File]
903576-44-3.mol | [Molecular Weight]
332 |
| Chemical Properties | Back Directory | [storage temp. ]
Desiccate at RT | [solubility ]
≥16.6 mg/mL in DMSO; ≥57.4 mg/mL in H2O; ≥94.2 mg/mL in EtOH | [form ]
solid | [color ]
White to off-white |
| Hazard Information | Back Directory | [Description]
Pitolisant hydrochloride,
a first-in-class inverse agonist of the histamine H3
receptor, was approved in the EU for the treatment of excessive
daytime sleepiness (EDS) in adults with narcolepsy with or
without cataplexy. The drug, which was developed by
Bioprojet and has orphan drug designation in the EU and
US, enhances wakefulness by increasing histaminergic neuron
activity. With once daily oral administration in the morning,
patients taking pitolisant exhibited significantly reduced EDS
versus placebo but not versus modafinil. Plasma levels of the
drug are reduced at the end of the day such that its waking
effect is minimized at night (plasma t1/2 10-12 h). Several
articles have been published detailing the discovery of
pitolisant. | [Uses]
BF 2649 Hydrochloride, is a novel histamine H3 receptor antagonist and inverse agonist. | [Synthesis]
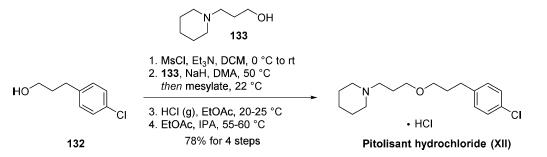
The most likely scale preparation of pitolisant hydrochloride
consists of only four synthetic steps starting with the mesylation
of commercial 3-(4-chlorophenyl)propan-1-ol (132). Displacement of the mesylate with the sodium salt of
commercial 3-(piperidin-1-yl)propan-1-ol (133) in warm DMA
assembled the parent drug in 97% yield over two steps. Salt
formation was affected by pH adjustment to 3-4 using HCl gas
in EtOAc. Recrystallization from ethyl acetate and isopropanol
provided pitolisant hydrochloride (XII) on kilogram scale in
78% overall yield across the short four-step protocol.
 | [in vitro]
bf2.649 behaved as a competitive antagonist with a ki value of 0.16 nm. bf2.649 functioned as an inverse agonist with an ec50 value of 1.5 nm and an intrinsic activity about 50% higher than that of ciproxifan. pitolisant in vitro potency was approximately 6 times lower at the rodent receptor [1]. | [in vivo]
pitolisant hcl was an oral bioavailable agonist. in mice, after oral and i.v. administrations of pitolisant hcl, the ratio of plasma areas under the curve was 84%. bf2.649 enhanced tele-methylhistamine levels in mouse brain, an index of histaminergic neuron activity in a dose dependent manner with an ed50 value of 1.6 mg/kg p.o. the response persisted after repeated administrations for 17 days [1]. treatment with 20-, 40-, or 60-mg doses of pitolisant showed a statistically significant suppressive effect (standardized photosensitive response [spr] reduction as measured with paired t-tests) in 9/14 (64%) patients of whom 6/14 (43%) showed abolition of the response to intermittent photic stimulation (ips) [3]. bf2.649 showed significant inhibitory activity in several mouse models of schizophrenia [4]. | [storage]
Desiccate at RT | [References]
[1] ligneau x, perrin d, landais l, camelin jc, calmels tp, et al, bf2. 649 [1-{3-[3-(4-chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine h3 receptor: preclinical pharmacology. j pharmacol exp ther.2007 jan;320(1):365-75.
[2] t a esbenshade, k e browman, r s bitner, m strakhova, m d cowart, j d brioni the histamine h3 receptor: an attractive target for the treatment of cognitive disorders. br j pharmacol. 2008 jul; 154(6): 1166–1181.
[3] kasteleijn-nolst trenité d, parain d, genton p, masnou p, schwartz jc, hirsch e. efficacy of the histamine 3 receptor (h3r) antagonist pitolisant (formerly known as tiprolisant; bf2.649) in epilepsy: dose-dependent effects in the human photosensitivity model. epilepsy behav.2013 jul;28(1):66-70.
[4] ligneau x, landais l, perrin d, piriou j, uguen m, denis e, robert p, parmentier r, anaclet c, lin js, burban a, arrang jm,schwartz jc. brain histamine and schizophrenia: potential therapeutic applications of h3-receptor inverse agonists studied with bf2.649. biochem pharmacol. 2007 apr 15;73(8):1215-24. a |
|
|