| Identification | Back Directory | [Name]
ROLAPITANT HYDROCHLORIDE | [CAS]
914462-92-3 | [Synonyms]
Rolapitant HCl hydrate
ROLAPITANT HYDROCHLORIDE
RolapitantMonohydrochloride
Rolapitant Hydrochloride Hydrate
Rolapitant Hydrochloride Monohydrate
(5S,8S)-8-[[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]methyl]-8-phenyl-1,9-diazaspiro[4.5]decan-2-one,hydrate,hydrochloride
1,7-Diazaspiro[4.5]decan-2-one, 8-[[(1R)-1-[3,5-bis(trifluoroMethyl)phenyl]ethoxy]Methyl]-8-phenyl-, Monohydrochloride, Monohydrate, (5S,8S)- | [EINECS(EC#)]
682-730-5 | [Molecular Formula]
C25H29ClF6N2O3 | [MDL Number]
MFCD23105917 | [MOL File]
914462-92-3.mol | [Molecular Weight]
554.96 |
| Chemical Properties | Back Directory | [Melting point ]
>149oC (dec.) | [storage temp. ]
Hygroscopic, -20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [color ]
White |
| Hazard Information | Back Directory | [Description]
Rolapitant hydrochloride hydrate, originally discovered by
Schering-Plough and later developed by TESARO, Inc., was
approved by the FDA in September 2015 for the prevention of
delayed chemotherapy-induced nausea and vomiting (CINV)
in combination with other antiemetic agents. Rolapitant is a
highly selective NK-1 receptor antagonist, exhibiting >1000-
fold selectivity for NK-1 over human NK-2 and NK-3 receptors
in vitro. In contrast to other NK-1 inhibitors that play an
essential role in delayed CINV therapy, rolapitant shows no
inhibition of CYP3A4, eliminating the need for concern when
coadministering with CYP34A substrates. Additionally, rolapitant
is an orally active agent with a relatively long half-life (180
h), providing potential opportunities for single- and
prechemotherapy-based treatments. In three large clinical
trials involving patients receiving moderately emetogenic
chemotherapy (MEC) and highly emetogenic chemotherapy
(HEC), subjects using rolapitant as a cotherapy with
granisetron and dexamethasone showed a significant improvement
in complete response compared to those receiving
treatments of granisetron and dexamethasone. | [Uses]
Rolapitant Hydrochloride Hydrate is a raw material for pharmaceutical formulations. | [Definition]
ChEBI: A hydrate that is the monohydrate form of rolapitant hydrochloride. Used for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy. | [Synthesis]
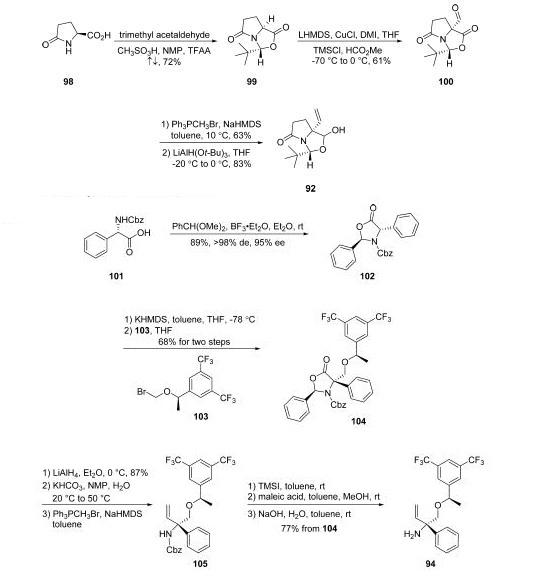
Rolapitant features a fascinating molecular architecture
consisting of two tetrasubstituted stereogenic carbon centers
situated at the 2- and 5-carbons within a central piperidine ring and a spirocyclic array residing at the 5-position and a phenyl
ring and ethereal linkage branching from the 2-position. The overall synthetic strategy to secure rolapitant
hydrochloride hydrate relies upon the union of two advanced
chiral building blocks that contain functional groups capable of
securing the central piperidine ring. These two key
intermediates, pyroglutamate derivative 93 and allylic amine
94, each bear one of the essential stereocenters embedded
within the structure of the active pharmaceutical ingredient.
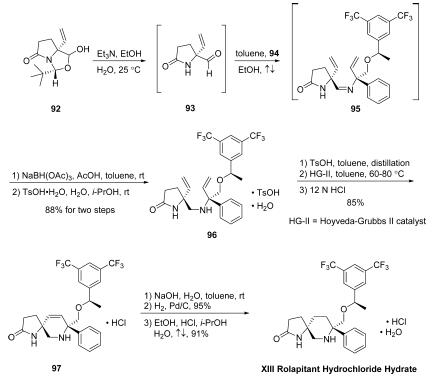
The first of these advanced intermediates, amidoaldehyde 93, is
generated directly by base-mediated decomposition of
pyroglutamic aminal 92. Subjection of 92 to triethylamine in
EtOH/H2O at ambient temperatures led to generation of chiral
allyl aldehyde 93, which was not isolated but condensed
immediately with amine 94 in the presence of
refluxing toluene to provide divinyl imine 95, which underwent
immediate reduction using NaBH(OAc)3 in AcOH/toluene to
furnish the free amine. The free amine was converted to the
corresponding tosylate monohydrate salt and triturated,
providing 96 as a white crystalline powder after subjection to
TsOH?¤H2O in i-PrOH/H2O. Divinyl amine 96 could then be
reacted with a solution of TsOH in toluene, distilled, and
directly combined with a toluene solution of Hoveyda-Grubbs
second-generation catalyst (HG-II) under heating conditions,
leading to the desired ring-closing metathesis product 97 as the
HCl salt (85% yield over two steps) after filtration, distillation,
and workup with 12N HCl. Washing of a toluene solution of 97
with aqueous NaOH and subsequent treatment of the resulting
organic solution with H2, wet Pd/C, and additional granular
activated carbon (Nuchar Aquaguard) led to the fully reduced
piperidine product in high yield (95%). Rolapitant hydrochloride
hydrate XIII was accessed thereafter by precipitation
from a solution of EtOH/i-PrOH/H2O/HCl, providing the
product as a white solid (91% yield).
 |
|
|