| Identification | Back Directory | [Name]
Rilmazafone | [CAS]
99593-25-6 | [Synonyms]
Rhythmy
450191-S
Rilmazafone
2',5-dichloro-2-(3-dimethylcarbamoyl-5-glycylaminomethyl-1H-1,2,4-triazol-1-yl)benzophenone
N,N-Dimethyl-1-[2-(2-chlorobenzoyl)-4-chlorophenyl]-5-(aminoacetylaminomethyl)-1H-1,2,4-triazole-3-carboxamide
5-[[(2-aminoacetyl)amino]methyl]-1-[4-chloro-2-(2-chlorobenzoyl)phenyl]-N,N-dimethyl-1,2,4-triazole-3-carboxamide
5-[[(Aminoacetyl)amino]methyl]-1-[4-chloro-2-(2-chlorobenzoyl)phenyl]-N,N-dimethyl-1H-1,2,4-triazole-3-earboxamide
5-[[(Aminoacetyl)amino]methyl]-1-[4-chloro-2-(2-chlorobenzoyl)phenyl]-N,N-dimethyl-1H-1,2,4-triazole-3-carboxamide
1H-1,2,4-Triazole-3-carboxamide, 5-[[(2-aminoacetyl)amino]methyl]-1-[4-chloro-2-(2-chlorobenzoyl)phenyl]-N,N-dimethyl- | [Molecular Formula]
C21H20Cl2N6O3 | [MDL Number]
MFCD00866989 | [MOL File]
99593-25-6.mol | [Molecular Weight]
475.33 |
| Chemical Properties | Back Directory | [density ]
1.45±0.1 g/cm3(Predicted) | [pka]
13.13±0.46(Predicted) | [InChI]
InChI=1S/C21H20Cl2N6O3/c1-28(2)21(32)20-26-17(11-25-18(30)10-24)29(27-20)16-8-7-12(22)9-14(16)19(31)13-5-3-4-6-15(13)23/h3-9H,10-11,24H2,1-2H3,(H,25,30) | [InChIKey]
KYHFRCPLIGODFH-UHFFFAOYSA-N | [SMILES]
N1(C2=CC=C(Cl)C=C2C(=O)C2=CC=CC=C2Cl)C(CNC(CN)=O)=NC(C(N(C)C)=O)=N1 |
| Hazard Information | Back Directory | [Description]
Rilmazafone is known as one of the 'Japanese benzos' because it was first developed in Japan. It is a substituted heterocyclic 1,2,4 triazole of the class triazolyl benzophenones.
Rilmazafone is considered to be a benzodiazepine pro-drug, and in Japan, is used for the short-term treatment of insomnia. Benzodiazepines are scheduled as Prescription Medicines in Schedule 1 of the Medicines Regulations 1984. However, rilmazafone is itself not a benzodiazepine. | [Uses]
Rilmazafone is a water-soluble benzodiazepine prodrug that acts as a sedative and hypnotic neuropsychiatric agent.
Nishino et al (2008) examined the anxiolytic effect of short-acting benzodiazepine hypnotics, including rilmazafone, in mice using an elevated plus-maze. They concluded that these were more potent, with smaller doses, than diazepam used as a control.
Rilmazafone has been considered to have a high potential to influence performance in the equine athlete (Kentucky Horse Racing Commission, 2015).
Classification of Rilmazafone | [Definition]
ChEBI: Rilmazafone is a member of benzophenones. | [Pharmacology]
Rilmafazone belongs to a group of open-ring benzodiazepinone analogs called peptide aminobenzophenones.It appears to be a precursor of the triazolobenzodiazepinones.It has both sedative and antianxiety effects. | [Synthesis]
Rilmafazone is prepared from 2,5-dichloro-2-aminobenzophenone as described in below:
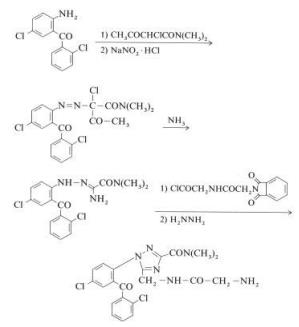 | [Metabolism]
Rilmazafone is not a naturally occurring metabolite and is only found in those individuals exposed to this compound or its derivatives.
Rilmazafone, were suggested to have more beneficial pharmacolog-ical properties in comparison to standard benzodiazepines.Rilmazafone is a ring-opened derivative of 1,4-benzodiazepine (Scheme 12.28) and was developed inJapan as an orally active sleep inducer.
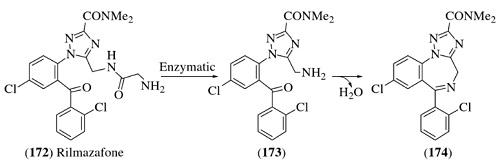
Rilmazafone is exclusively metabolized byaminopeptidases in the small intestine to the labile desglycylated metabolite 173and then to its cyclic form 174.The concentration of 174 in the systemic plasma (i.e., bioavailability) after oral administration of rilmazafone has been reported to behigher than that observed after administration of 174 due to the lower hepatic extrac-tion of 173 than 174. |
|