1421693-22-2
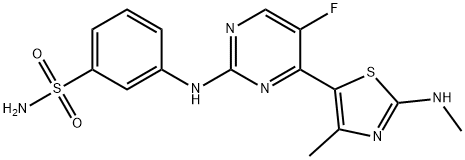 1421693-22-2 结构式
1421693-22-2 结构式
基本信息
CDKI-73
CDKI-73 (CDKI73)
Benzenesulfonamide, 3-[[5-fluoro-4-[4-methyl-2-(methylamino)-5-thiazolyl]-2-pyrimidinyl]amino]-
物理化学性质
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/02/08 | HY-12445 | Asnuciclib | 1421693-22-2 | 1 mg | 1600元 |
| 2025/02/08 | HY-12445 | CS-2259 CDKI-73 | 1421693-22-2 | 2mg | 2333元 |
| 2025/02/08 | HY-12445 | CS-2259 CDKI-73 | 1421693-22-2 | 5mg | 3500元 |
常见问题列表
| Target | Value |
|
CDK2
(Cell-free assay) | 3.27 nM |
|
CDK9
(Cell-free assay) | 5.78 nM |
|
CDK1
(Cell-free assay) | 8.17 nM |
|
CDK4
(Cell-free assay) | 8.18 nM |
CDKI-73 is highly cytotoxic to primary leukemia cells derived from CLL patients (mean LD
50
= 0.08 μM) and shows>500-fold selectivity for primary leukemia cells over normal B-lymphocytes (LD
50
= 40.5 μM).
CDKI-73 (0.1 μM, 4 h) inhibits the phosphorylation of serine 2 of RNA polymerase II and MCL1 protein expression in CLL cells.
CDKI-73 induced caspase-dependent apoptosis that was preceded by dephosphorylation of cdk9 and serine 2 of RNA polymerase II.
CDKI-73 is highly effective against all cell lines tested with an IC
50
in the range of 0.012-0.517 μM; in particular three MLL-AML cell lines, namely MOLM13, MV4-11 and THP-1, were highly sensitive to CDKI-73 with IC
50
values <0.062 μM.
Cell Viability Assay.
| Cell Line: | CLL cells. |
| Concentration: | 0-1 μM. |
| Incubation Time: | 48 h. |
| Result: | Shows preferential cytotoxicity in CLL cells. |
CDKI-73 (25, 50, 100 mg/kg) markedly decreases tumor growth in a dose-dependent manner and results in a prolongation of animal life span (P < 0.001) without causing body weight loss and other overt toxicities..
| Animal Model: | MV4-11 tumor bearing mice. |
| Dosage: | 25 mg/kg. |
| Administration: | Orally once everyday for 33 days. |
| Result: | Caused a remarkable delay in tumor growth compared to vehicle-treated mice, as reflected in a percentage for the mean tumor volume in treated to control mice of 43% at day 31. |
| Animal Model: | Balb/C mice aged 6-8 weeks. |
| Dosage: | 2 mg/kg (IV), 10, 20 and 40 mg/kg (PO). (Pharmacokinetic Analysis.) |
| Administration: | IV and PO, single dose. |
| Result: |
The C
max
increased from 1.29 to 3.66 μM at a mean time of 1 h and the area under the curve (AUC) of CDKI-73 increased from 3.51 to 12.8 μM.h when the oral dose was escalated from 10 to 40 mg/kg.
CDKI-73 was eliminated from plasma with a mean terminal half-life (T1/2) of 2 h. Its oral bioavailability (F) ranged from 54 to 85% across the three doses. |