352458-37-8
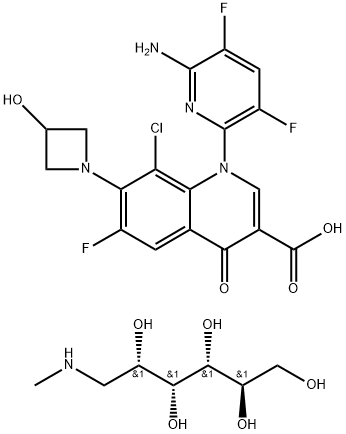 352458-37-8 结构式
352458-37-8 结构式
基本信息
德拉沙星葡甲胺
地拉沙星葡甲胺
德拉沙星葡甲胺盐
352458-37-8
德拉沙星N-甲基葡萄糖胺盐
DELAFLOXACIN葡甲胺盐
ABT492 MegluMine
ABT 492 MegluMine
ABT-492 MegluMine
RX-3341 meglumine
ABT-491 MegluMine
WQ-3034 meglumine)
Delafloxcain meglumine
Delafloxacin Mcglumine
Delafloxacin (MegluMine)
物理化学性质
常见问题列表
德拉沙星葡甲胺(delafloxacin meglumine)由日本湧永制药株式会社(Wakunaga Pharmaceutical Co. Ltd.)研制,1996年授予美国RIB-X制药公司全球独家开发权,由该公司旗下Melinta生物制药公司全权负责研发、上市、生产和销售。美国参议院为激励抗菌药物的研发,于2012年6月26日审议通过美国抗菌药物研发法案,即《食品药品管理局安全与创新法案》(FDASIA)。2014年尚处于Ⅲ期临床试验的德拉沙星葡甲胺符合美国食品药品管理局(FDA)制定的"合格的抗感染药品"(QIDP)认证条件,从而获得优先审评待遇,于2017年6月19日获准上市,商品名为Baxdela。
Antibiotic
Delafloxacin (the total daily doses vary from 0.156 to 640 mg/kg/24 h, subcutaneous injection) is highly effective against
S. aureus
. Against all four strains are observed a decrease of 1.5 to 2.2 log
10
CFU in organism burden from untreated controls at even the lowest dose studied, and for two strains (MW2 and R2527) there is net bactericidal activity at the lowest dose. At the maximal doses studied, there is a >4-log
10
kill from initial burden for all
S. aureus
strains.
Delafloxacin (2.5, 10, 40, and 160 mg/kg; subcutaneous injection, 24 h) has moderate terminal elimination half-life (t
1/2
=0.68 h, 0.79 h, 0.69 h and 1.0 h for 2.5 mg/kg, 10 mg/kg, 40 mg/kg, and 160 mg/kg, respectively).
| Animal Model: | Mice with a neutropenic murine lung infection model (four S. aureus , four S. pneumoniae , and four K. pneumoniae strains) |
| Dosage: | The total daily doses vary from 0.156 to 640 mg/kg/24 h |
| Administration: | 0.03 to 160 mg/kg are administered every 6 h (q6h) to infected mice by subcutaneous injection |
| Result: |
Inhibited
S. aureus
strains ATCC 29213, ATCC 33591, MW2, R2527 with MICs of 0.008, 0.008, 0.004, and 0.004 mg/L, respectively.
Inhibited S. pneumoniae strains ATCC 10813, ATCC 49619, 145, and 1329 with MICs of 0.03, 0.125, 0.016, and 0.016 mg/L, respectively. Inhibited K. pneumonia strains ATCC 43816, 4105, 4110, and 81-1260A with MICs of 0.06, 1, 0.5, and 0.06 mg/L, respectively. |
| Animal Model: | Neutropenic mice |
| Dosage: | 2.5, 10, 40, and 160 mg/kg; 0.2 mL |
| Administration: | Subcutaneous injection; 24 h |
| Result: | The maximum drug concentrations (C max ) concentrations ranged from 2 to 71 mg/L. AUC 0-∞ values ranged from 2.8 to 152 mg•h/L and were linear across the 2.5- to 160-mg dosing range. The elimination half-life (t 1/2 ) ranged from 0.7 to 1 h. |