Disulfur trioxide Chemische Eigenschaften,Einsatz,Produktion Methoden
Synthese
S + SO3 = S2O3
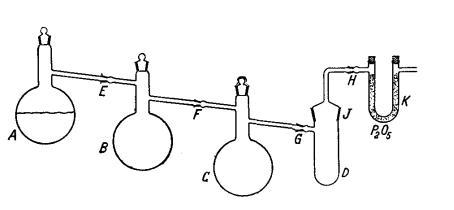
The Pyrex apparatus (Fig. 165) consists of distillation flasks
A, B and 0 (300 ml. each), reaction vessel V, and U tube A', filled
with a P3O5 -glass wool mixture. About 1 g. of
carefully purified S is charged into vessel D through
ground glass joint J, and 200 ml. of pure, 65% oleum is placed in
the flask A. Then A is heated very slowly in an H3SO4 bath while
B is cooled in an ice-water bath and Q with an ice-salt bath. Most
of the SO3 condenses in B and only a small amount passes into Q.
As soon as all the SO3 which can be removed from the acid by
gentle heating is evaporated, flask A is allowed to cool and is then disconnected at joint E; the latter is immediately closed off. About
three quarters of the SO3 in B is then distilled into 0 in the same
manner by cooling 0 with ice water and D with an ice-salt mixture.
Flask B is then disconnected at F. If the product collected in 0 is
not yet sufficiently pure (it should melt at 15-16°C to a colorless
liquid), it must be redistilled using flasks A and B (which are
meanwhile cleaned and dried). To achieve the reaction, the SO3 in
0 is heated until it melts, and 15 ml. of the melt is allowed to
deposit on the sulfur in D by rotating the flask in the ground glass
joint G. The mixture instantaneously turns a deep blue. A vigorous
reaction starts after 30 seconds, evolving white vapors. It is then
advisable to disconnect the P3O5 tube at H from time to time. It is
important to maintain the flask contents at approximately 15°C at
all times. Above that temperature, the product S3O3is markedly
decomposed, while at lower temperatures the excess SO3 solidifies.
If this happens, separation of the SO3 becomes very difficult and is
accompanied by partial decomposition of the S3O3.
Disulfur trioxide Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

