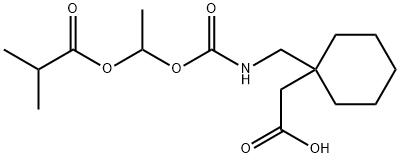
Gabapentin enacarbil
- CAS No.
- 478296-72-9
- Chemical Name:
- Gabapentin enacarbil
- Synonyms
- XP 13512;GABAPENTIN ENACARBIL;Gabapentin Enarcarbil;Gabapentin Impurity 11;Gabapentin enacarbil (XP-13512);TIANFUCHEM-478296-72-9---Gabapentin enacarbil;1-[[[[1-(2-Methyl-1-oxopropoxy)ethoxy]carbonyl]amino]methyl]...;2-(1-((((1-(Isobutyryloxy)ethoxy)carbonyl)amino)methyl)cyclohexyl)acetic acid;1-[[[[1-(2-Methyl-1-oxopropoxy)ethoxy]carbonyl]amino]methyl]cyclohexaneacetic acid;Cyclohexaneacetic acid,1-[[[[1-(2-Methyl-1-oxopropoxy)ethoxy]carbonyl]aMino]Methyl]-
- CBNumber:
- CB01011737
- Molecular Formula:
- C16H27NO6
- Molecular Weight:
- 329.39
- MDL Number:
- MFCD09954124
- MOL File:
- 478296-72-9.mol
| Melting point | 65 °C |
|---|---|
| Boiling point | 482.0±20.0 °C(Predicted) |
| Density | 1.134 |
| storage temp. | Store at -20°C |
| solubility |
DMSO:100.0(Max Conc. mg/mL);303.59(Max Conc. mM) Ethanol:100.0(Max Conc. mg/mL);303.59(Max Conc. mM) Water:0.67(Max Conc. mg/mL);2.03(Max Conc. mM) |
| form | Powder |
| pka | 4.72±0.10(Predicted) |
| color | Light yellow to brown |
| CAS DataBase Reference | 478296-72-9(CAS DataBase Reference) |
| FDA UNII | 75OCL1SPBQ |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
Gabapentin enacarbil price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ChemScene | CS-1698 | Gabapentinenacarbil | 478296-72-9 | 10mg | $300 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FG31551 | Gabapentin enacarbil | 478296-72-9 | 25mg | $300 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FG31551 | Gabapentin enacarbil | 478296-72-9 | 50mg | $350 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0008802 | GABAPENTIN ENACARBIL 95.00% | 478296-72-9 | 1G | $624.75 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FG31551 | Gabapentin enacarbil | 478296-72-9 | 2mg | $80 | 2021-12-16 | Buy |
Gabapentin enacarbil Chemical Properties,Uses,Production
Description
Gabapentin enacarbil (GEn) is an actively transported prodrug of gabapentin that provides sustained doseproportional exposure to gabapentin and predictable bioavailability. In April 2011, Gabapentin enacarbil is approved by the US Food and Drug Administration for the treatment of moderate-to-severe primary restless legssyndrome (RLS) in adults.
Gabapentin enacarbil was designed to be recognized as a substrate for two high-capacity nutrient transports, monocarboxylate transporter type 1 and sodium-dependent multivitamin transporter, and to be efficiently cleaved after absorption to give gabapentin. The separated enantiomers of gabapentin enacarbil have similar cleavage rates in human tissues. Preclinical studies showed that gabapentin enacarbil provides good systemic exposure of gabapentin in rats and monkeys.
Originator
Xenoport (United States)
Uses
Gabapentin enacarbil (HORIZANT GlaxoSmithKline/XenoPort) is a prodrug of gabapentin used as an anticonvulsant as well as a treatment for neurogenic pain, with the same mechanism of action as pregabalin.
Definition
ChEBI: A carbamate ester that is the N-[1-(isobutyryloxy)ethoxy]carbonyl derivative of [1-(aminomethyl)cyclohexyl]acetic acid. The prodrug for gabapentin, used for treatment of neuropathic pain and restless legs syndrome.
Preparation
Gabapentin enacarbil is prepared as a racemic mixture from gabapentin either by sequential coupling with 1-chloroethyl chloroformate in the presence of trimethylsilyl chloride and triethylamine followed by addition of isobutyric acid or by direct coupling with an activated 1-(isobutyryloxy) ethyl carbonate.
brand name
Horizant
Pharmacokinetics
Gabapentin enacarbil is an acyloxyalkylcarbamate prodrug of analgesic and anticonvulsant drug gabapentin which has problematic pharmacokinetic properties, including short half-life, saturable absorption, high inter-patient variability, and lack of linear dose–response relationship. Gabapentin enacarbil was designed to be absorbed throughout the entire length of the gastrointestinal tract, and its absorption is mediated by high-capacity nutrient transporters, including monocarboxylate transporter 1 (MCT-1) and sodium-dependent multivitamin transporter (SMVT). Prodrug modification produced an extended release of gabapentin with twofold improved, more predictable, and dose-proportional oral bioavailability in humans. During and after its absorption, gabapentin enacarbil is efficiently hydrolyzed by nonspecific esterases to yield gabapentin. Currently, gabapentin enacarbil is commercially available for the treatment of restless legs syndrome and post-herpetic neuralgia of adults.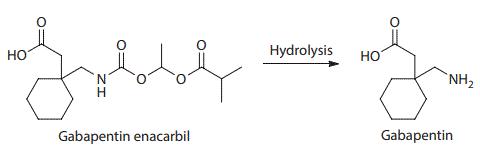
Structure and hydrolysis of gabapentin enacarbil to the active gabapentin
Clinical Use
Gabapentin enacarbil is a prodrug of gabapentin (Neurontin, Pfizer) which binds to the a2-d subunit of L-type voltage-regulated calcium channels, reducing the release of several neurotransmitters. 122,123 Gabapentin enacarbil was discovered at XenoPort, codeveloped with GlaxoSmithKline, is marketed under the brand name Horizant, and is approved for the treatment of moderate to severe restless leg syndrome. Gabapentin enacarbil was designed to increase the absorption of gabapentin through the interaction with sodium-dependent multivitamin transporter (SMVT) and monocarboxylate transporter type 1 (MCT-1). As a result, the drug demonstrated much better oral bioavailability and more consistent exposure compared to the parent.
Synthesis
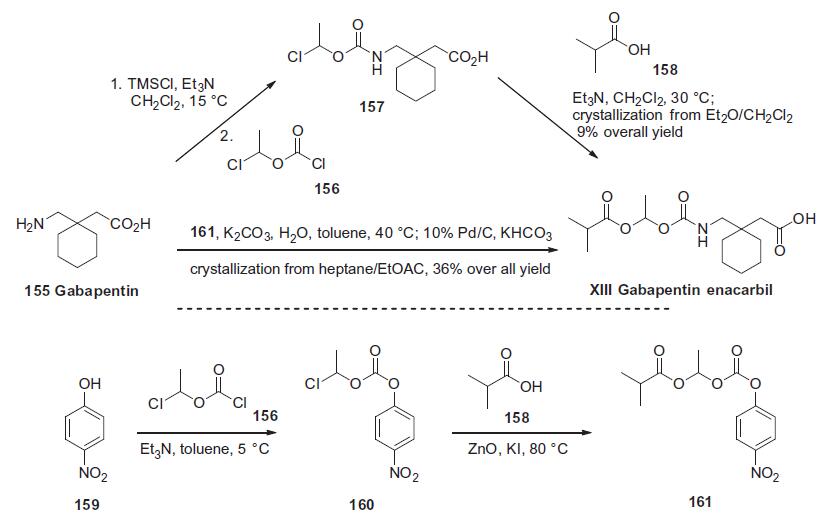
Gabapentin 155 was treated with chlorotrimethylsilane and triethylamine followed by acylation with 1-chloroethyl chloroformate 156 to give acid 157 after hydrolysis of the intermediate silyl ester. This acid was then used without purification and reacted with isobutyric acid (158) and triethylamine to afford gabapentin enacarbil (XIII) in 9.1% overall yield after crystallization. Alternatively, gabapentin 155 was reacted directly with the fully elaborated p-nitrophenyl activated side chain 161 in the presence of potassium carbonate. The resulting mixture of products and p-nitrophenol was treated with 10% Pd/C and potassium formate followed by acidic workup to remove the resulting aniline, providing gabaentin enacarbil (XIII) in 36% overall yield from p-nitrophenol 159 after crystallization. The required activated side chain 161 was prepared from p-nitrophenol 159 via a two-step, one-pot process involving acylation of the phenol with 1-chloroethyl chloroformate 156 in triethylamine. This provided intermediate 160 which was alkylated with isobutyric acid (158) in the presence of zinc oxide and potassium iodide, ultimately furnishing the mixed carbonate 161.
Mode of action
Gabapentin enacarbil is a prodrug of gabapentin and, accordingly, its therapeutic effects in RLS and PHN are attributable to gabapentin.
The mechanism of action by which gabapentin is efficacious in PHN is unknown but in animal models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to painful stimuli). Gabapentin prevents pain-related responses in several models of neuropathic pain in rats and mice (e.g., spinal nerve ligation models, spinal cord injury model, acute herpes zoster infection model). Gabapentin also decreases pain-related responses after peripheral inflammation (carrageenan footpad test, late phase of formalin test), but does not alter immediate pain-related behaviors (rat tail flick test, formalin footpad acute phase). The relevance of these models to human pain is not known.
Gabapentin enacarbil Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12837 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32161 | 58 |
| Xi'an MC Biotech, Co., Ltd. | 029-89275612 +8618991951683 | mcbio_sales@163.com | China | 2251 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | China | 52849 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
View Lastest Price from Gabapentin enacarbil manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-01-31 | Gabapentin enacarbil
478296-72-9
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2021-08-11 | Gabapentin enacarbil
478296-72-9
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2019-07-06 | Gabapentin enacarbil
478296-72-9
|
US $2.00 / kg | 1kg | 99% | ask | Career Henan Chemical Co |
-

- Gabapentin enacarbil
478296-72-9
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Gabapentin enacarbil
478296-72-9
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Gabapentin enacarbil
478296-72-9
- US $2.00 / kg
- 99%
- Career Henan Chemical Co